Chemical Transformation
-
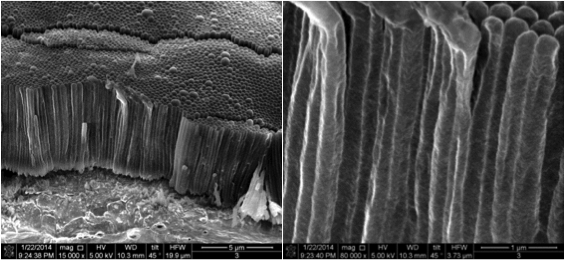
Dendrite-Free Lithium Deposition with Self-Aligned Nanorod Structure
Using an electrolyte additive that can suppress Li dendrite formation and improve the performance of Li metal batteries, we have achieved for the first time the growth of dendrite-free lithium films with self-aligned and highly-compacted nanorod structure. Read More
-
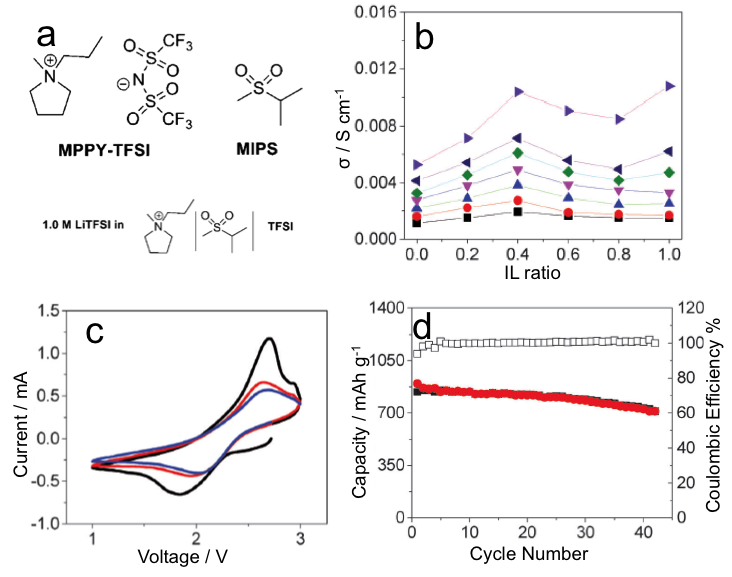
Synergistic Effects of Mixing Sulfone and Ionic Liquid as Safe Electrolytes for Lithium Sulfur Batteries
A strategy of mixing both an ionic liquid and sulfone is applied in Li-S batteries to give synergistic effects of reducing viscosity, increasing ionic conductivity, reducing polysulfide dissolution, and improving safety. Read More
-
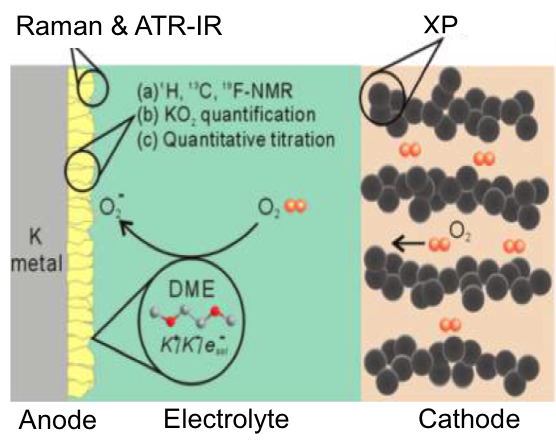
Understanding Side Reactions in K-O2 Batteries for Improved Cycle Life: a Combined DFT and Experimental Study
First comprehensive study of side reactions that dictate the fundamental electrochemistry of K-O2 battery has been carried out Read More
-
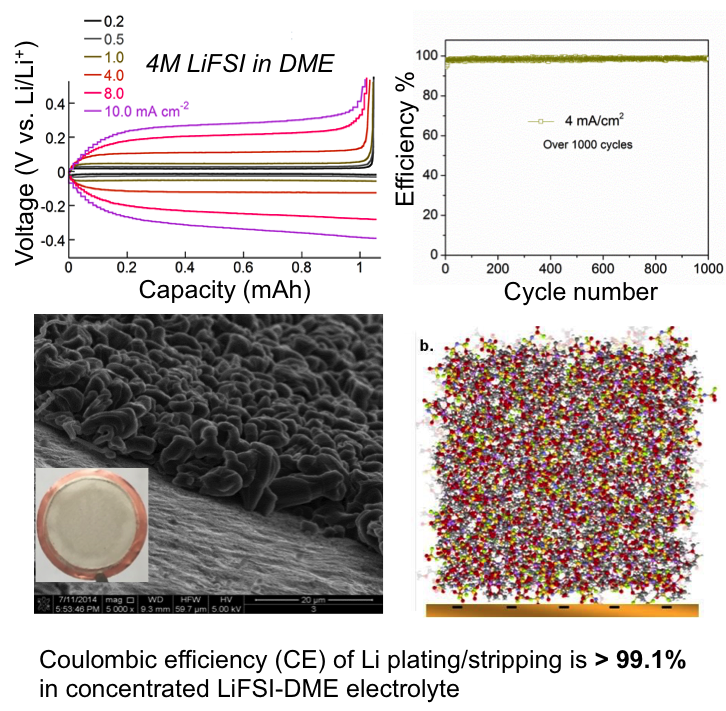
High Rate and Stable Cycling of Lithium Metal Anode
Lithium metal is an ideal battery anode. However, dendrite growth and limited CE during cycling have limited its practical applications. High CE (up to 99.1%) without dendrite growth is achieved by using highly concentrated electrolytes for lithium plating/stripping. Read More
-
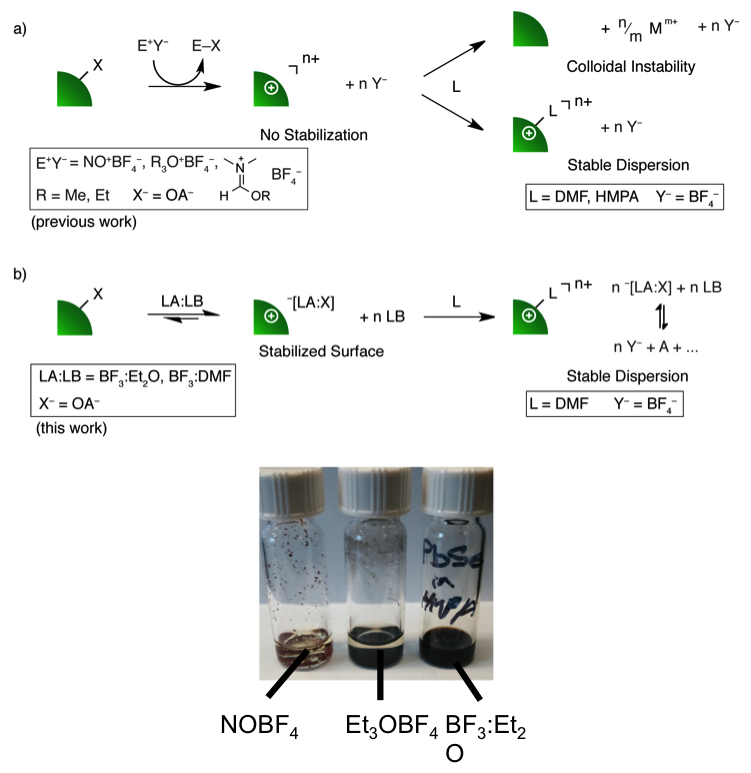
Mechanistic Insight into the Formation of Cationic Naked Nanocrystals Generated Under Equilibrium Control
Developed a class of ligand-stripping reagents that stabilize the nanocrystal (NC) surface during the stripping reaction, leading to improved dispersibility. Read More
-
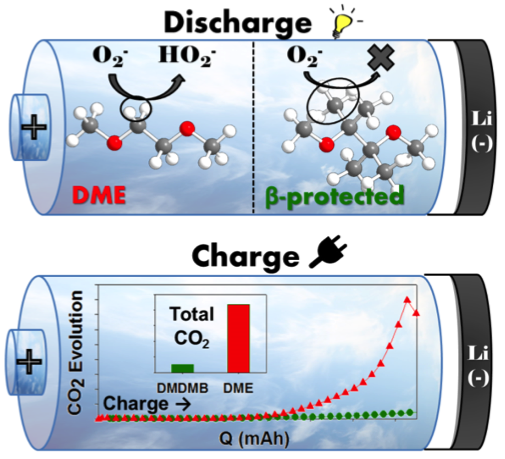
Towards a Stable Organic Electrolyte for Li-O2 Batteries
The precise decomposition pathways of glyme solvents were identified. Read More
-
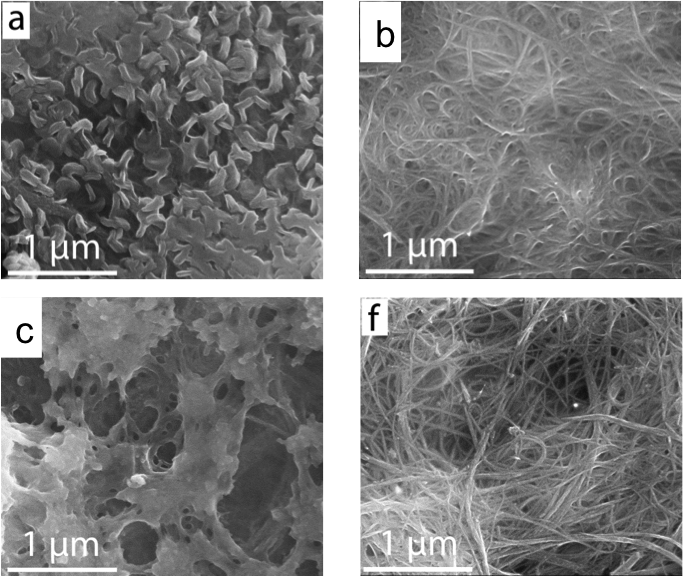
Formation of Interfacial Layer and Long-Term Cyclability of Li-O2 Batteries
Identified key factors that affect the long term cycle life of Li-O2 batteries under full discharge/charge conditions. Read More
-
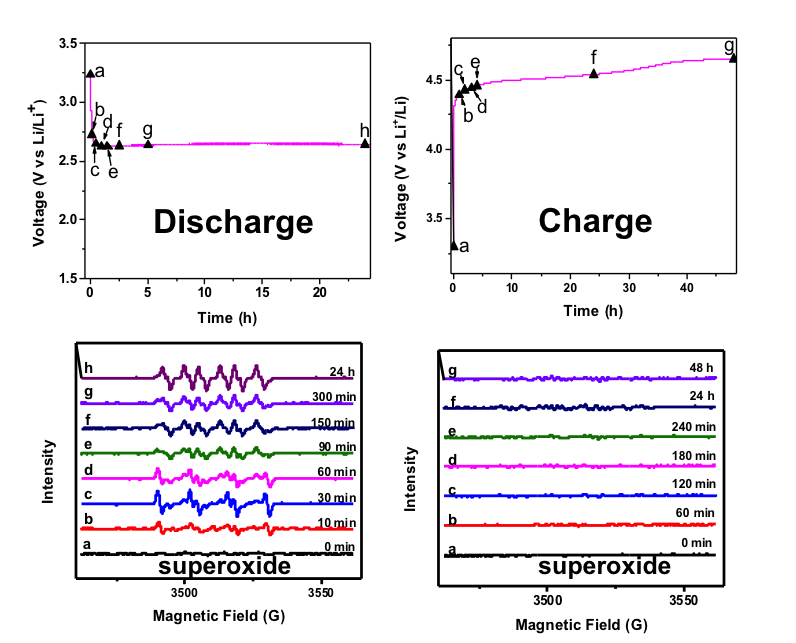
The mechanisms of oxygen reduction and evolution reactions in nonaqueous lithium-oxygen batteries
Superoxide radical anions (O2•−) are intermediates formed in non-aqueous lithium-oxygen batteries, which are very reactive but have a very short lifetime. Using 5,5-dimethyl-pyrroline N-oxide as a spin trap, we stabilized these radicals and enabled direct verification of superoxide radicals by electron paramagnetic resonance (EPR). Read More
-
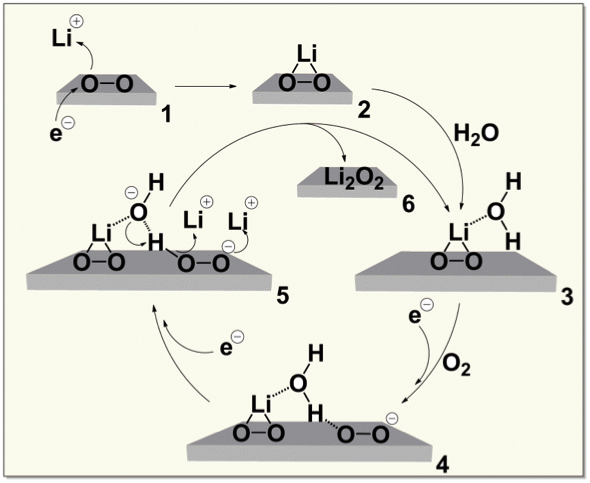
Trace Water Catalyzes Lithium Peroxide Electrochemistry
Water at ppm levels catalyzes the conversion of lithium superoxide (LiO2) to lithium peroxide (Li2O2) by the reaction cycle shown. Because water is not consumed in the cycle, trace amounts leverage large effects. Read More
-
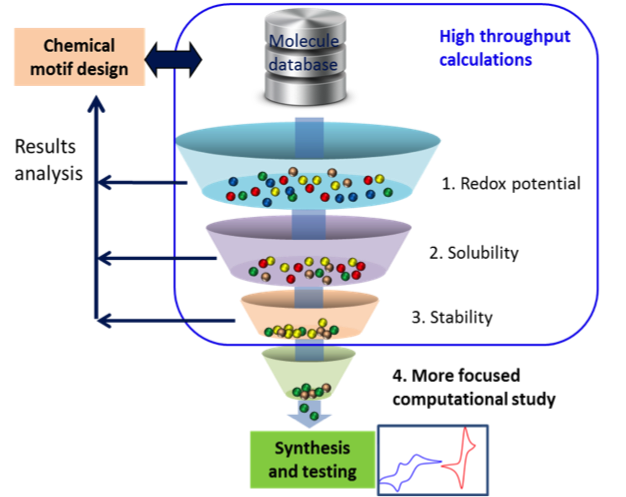
Realizing the Electrolyte Genome
JCESR is building a highly sophisticated infrastructure for high-throughput evaluation of molecular properties. This involves coupling ab initio DFT methods with rapid classic molecular dynamics, calculations of redox potentials, and solvation structure while more detailed reactivity studies are carried out. Candidates that meet performance metrics for different types of battery applications (redox-flow, Li-air, Li-sulfur and multivalent) are prioritized for synthesis and testing. Read More
Latest Updates
-
You’re Invited - JCESR and Beyond: Translating the Basic Science of Batteries
Please join us at Argonne National Laboratory on Tuesday, April 4, 2023 for JCESR and Beyond: Translating the Basic Science of Batteries. Registration is now open. This in-person event will celebrate 10 years of research from the Joint Center… Read More
-
A Message from JCESR: In Memory of George Crabtree
It is with heavy hearts that we say goodbye to George Crabtree, a Senior Scientist and Distinguished Fellow at Argonne National Laboratory, and Director of the Joint Center for Energy Storage Research (JCESR), who passed away unexpectedly on January 23. Dr. Read More
-
Cyanopyridines As Extremely Low-Reduction-Potential Anolytes for Nonaqueous Redox Flow Batteries
Discovery of a cyanophenylpyridine derivative with a very low reduction potential and good stability during cycling. Read More
-
Characterizing Redoxmer – Electrode Kinetics Using a SECM-Based Spot Analysis Method
Identified asymmetries in electron transfer (ET) kinetics between the reduction and oxidation of ferrocene-based redoxmers by measuring the ET rate constants (kf/kb) as a function of electrode potential. Read More
-
Benzotriazoles as Low Potential Anolytes for Non-Aqueous Redox Flow Batteries
We developed an easy-to-synthesize benzotriazole-based anolyte with a high energy redox potential (-2.3 V vs Fc/Fc+) and high solubility that demonstrates stable electrochemical cycling performance. Read More

