Research Highlights
-
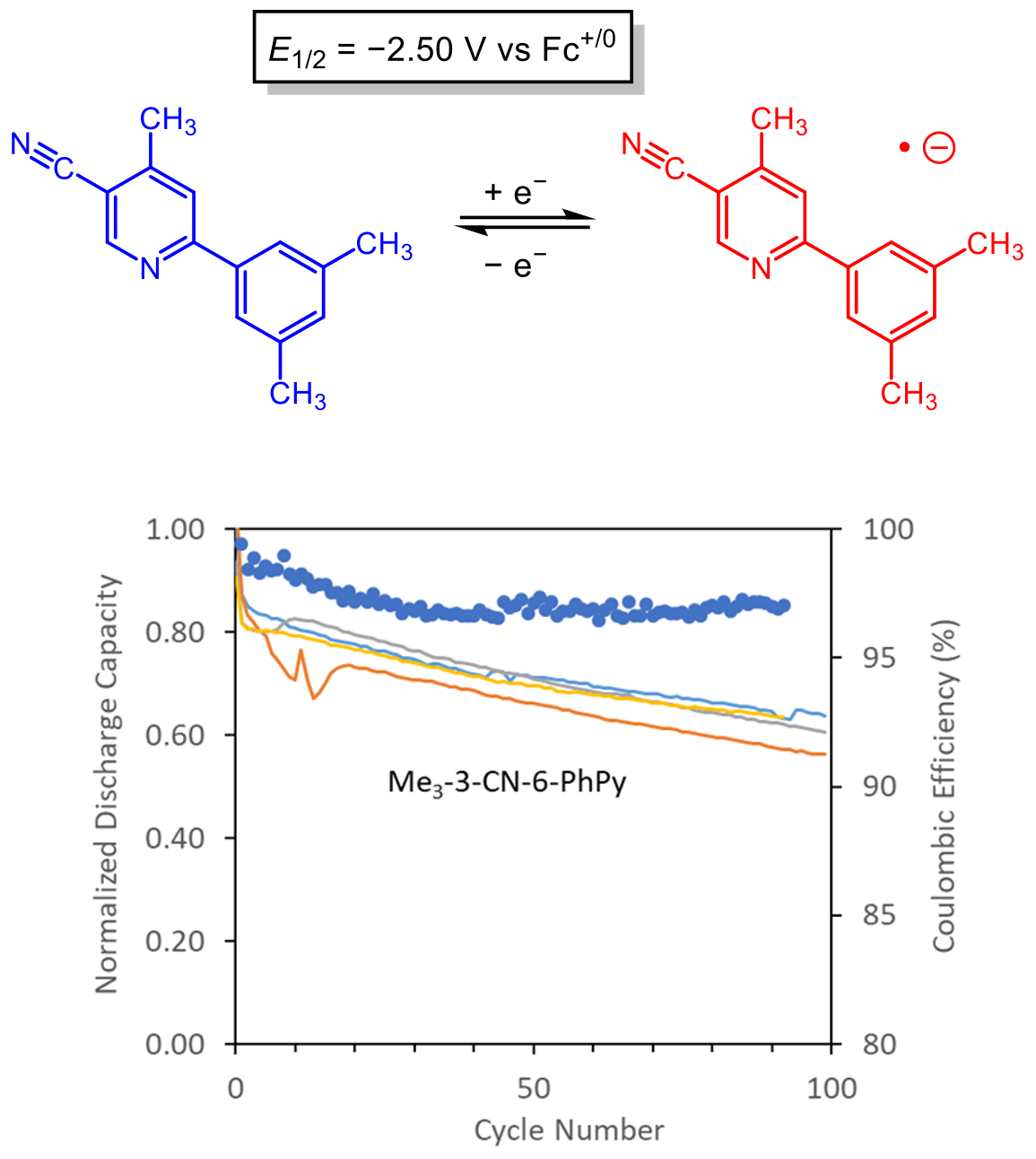
Cyanopyridines As Extremely Low-Reduction-Potential Anolytes for Nonaqueous Redox Flow Batteries
Discovery of a cyanophenylpyridine derivative with a very low reduction potential and good stability during cycling. Read More
-
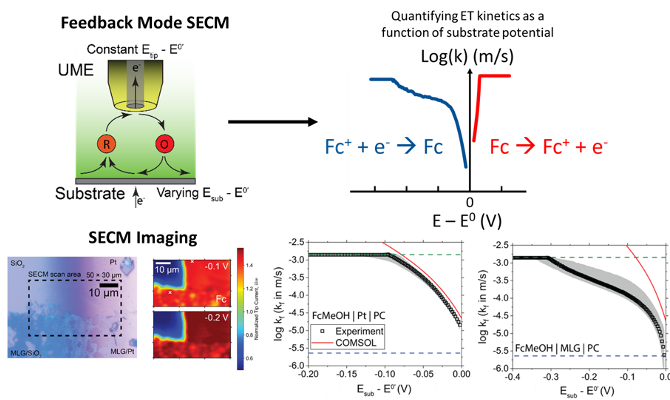
Characterizing Redoxmer – Electrode Kinetics Using a SECM-Based Spot Analysis Method
Identified asymmetries in electron transfer (ET) kinetics between the reduction and oxidation of ferrocene-based redoxmers by measuring the ET rate constants (kf/kb) as a function of electrode potential. Read More
-
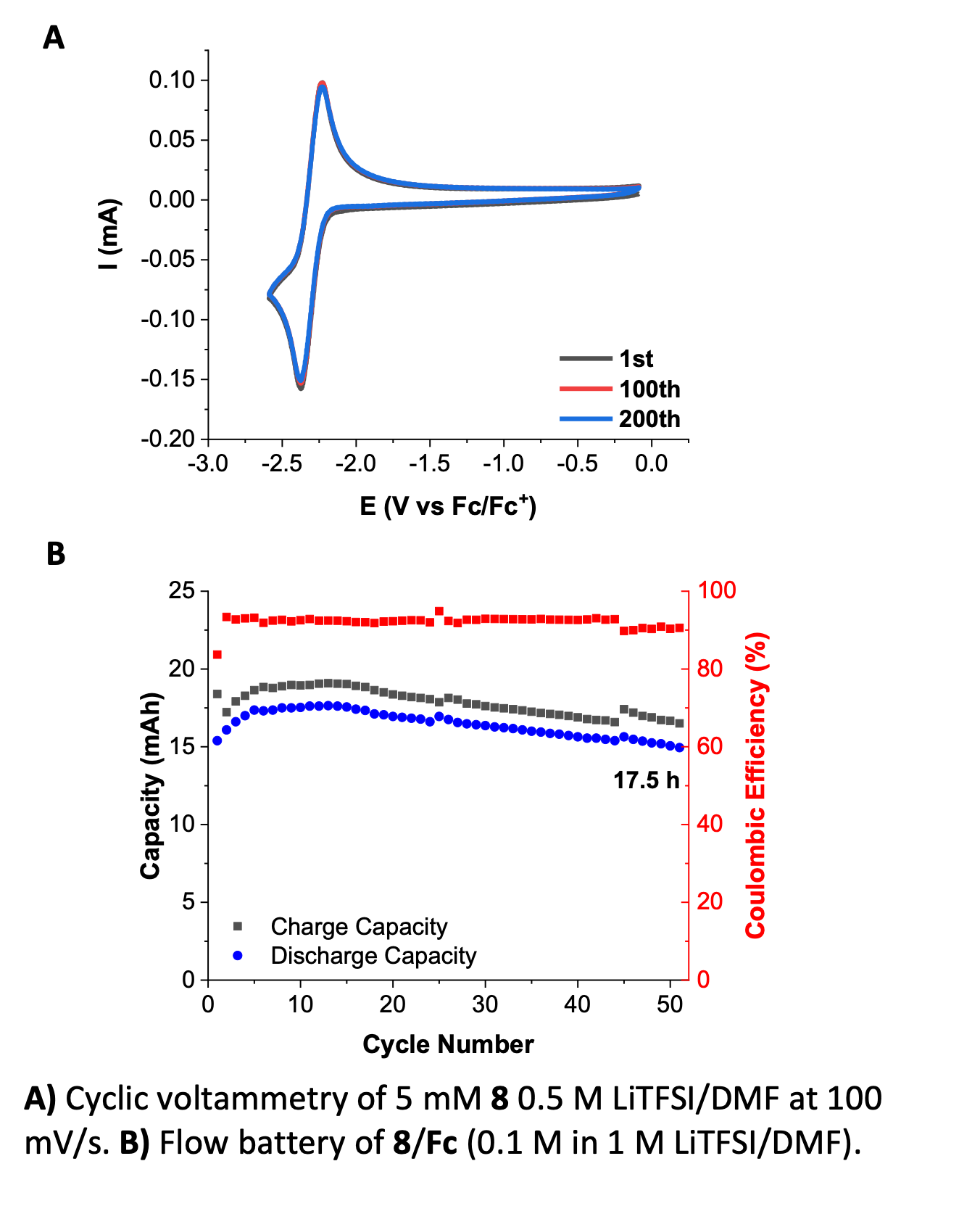
Benzotriazoles as Low Potential Anolytes for Non-Aqueous Redox Flow Batteries
We developed an easy-to-synthesize benzotriazole-based anolyte with a high energy redox potential (-2.3 V vs Fc/Fc+) and high solubility that demonstrates stable electrochemical cycling performance. Read More
-
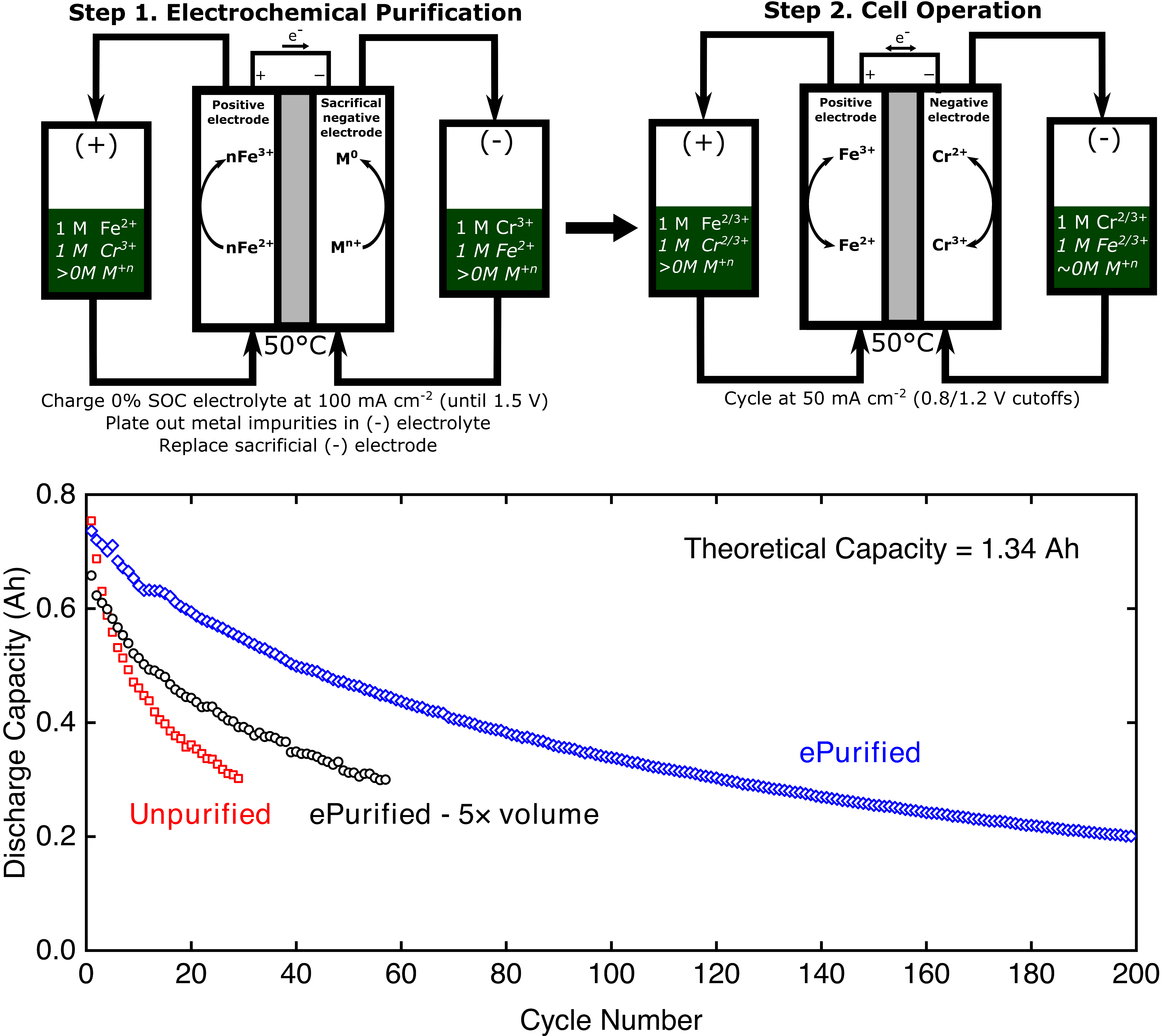
Hydrogen evolution mitigation in iron-chromium redox flow batteries via electrochemical purification of the electrolyte
An electrochemical purification method is used to mitigate the hydrogen evolution reaction (HER) in iron-chromium (Fe-Cr) redox flow batteries (RFBs), resulting in reduced capacity fade rates. Read More
-
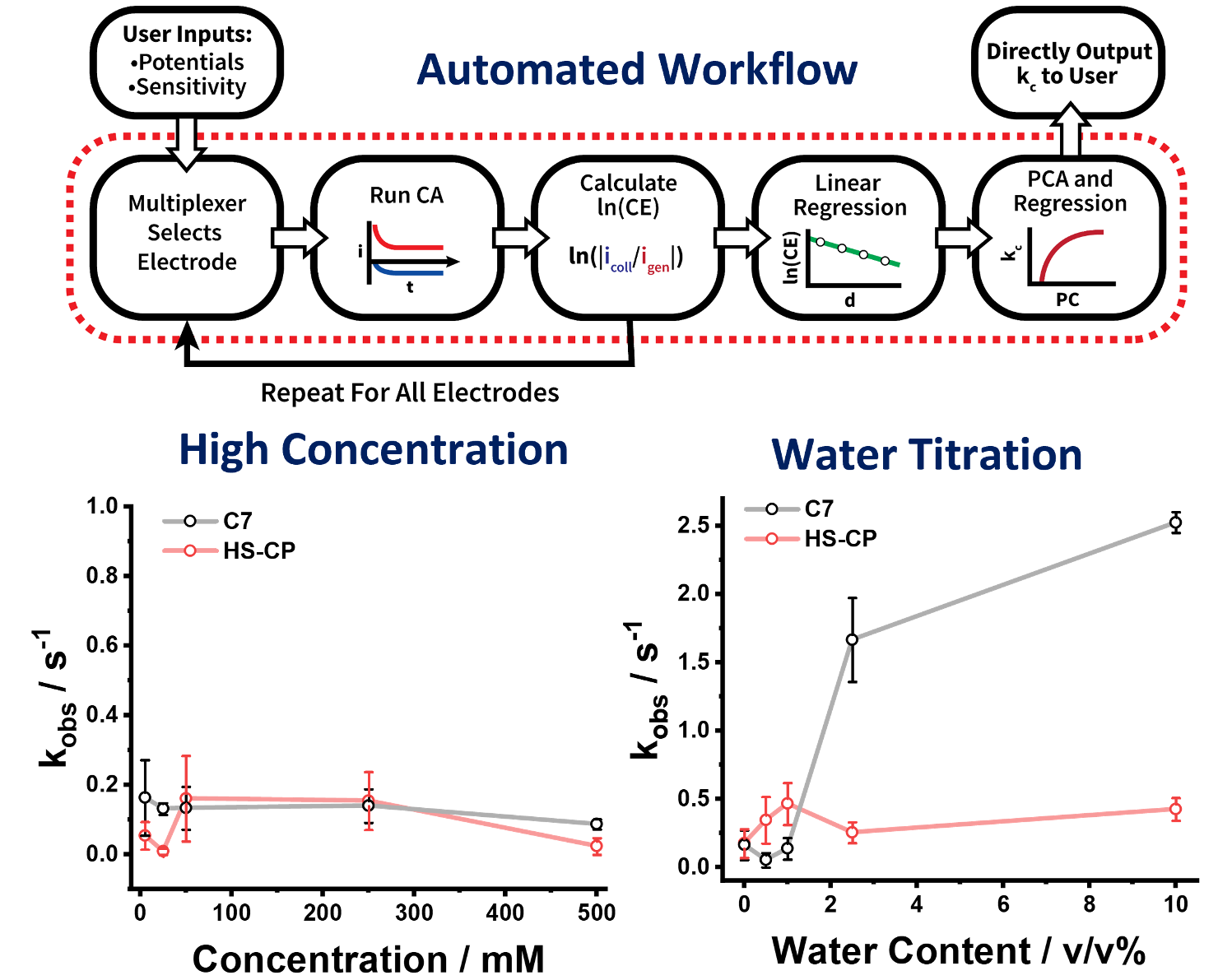
Automated Measurement of Electrogenerated Redox Species Degradation Using Multiplexed Interdigitated Electrode Arrays
Microfabricated devices enabled an automated methodology to quickly screen redoxmer degradation kinetics at concentrations up to 0.5 M. Read More
-
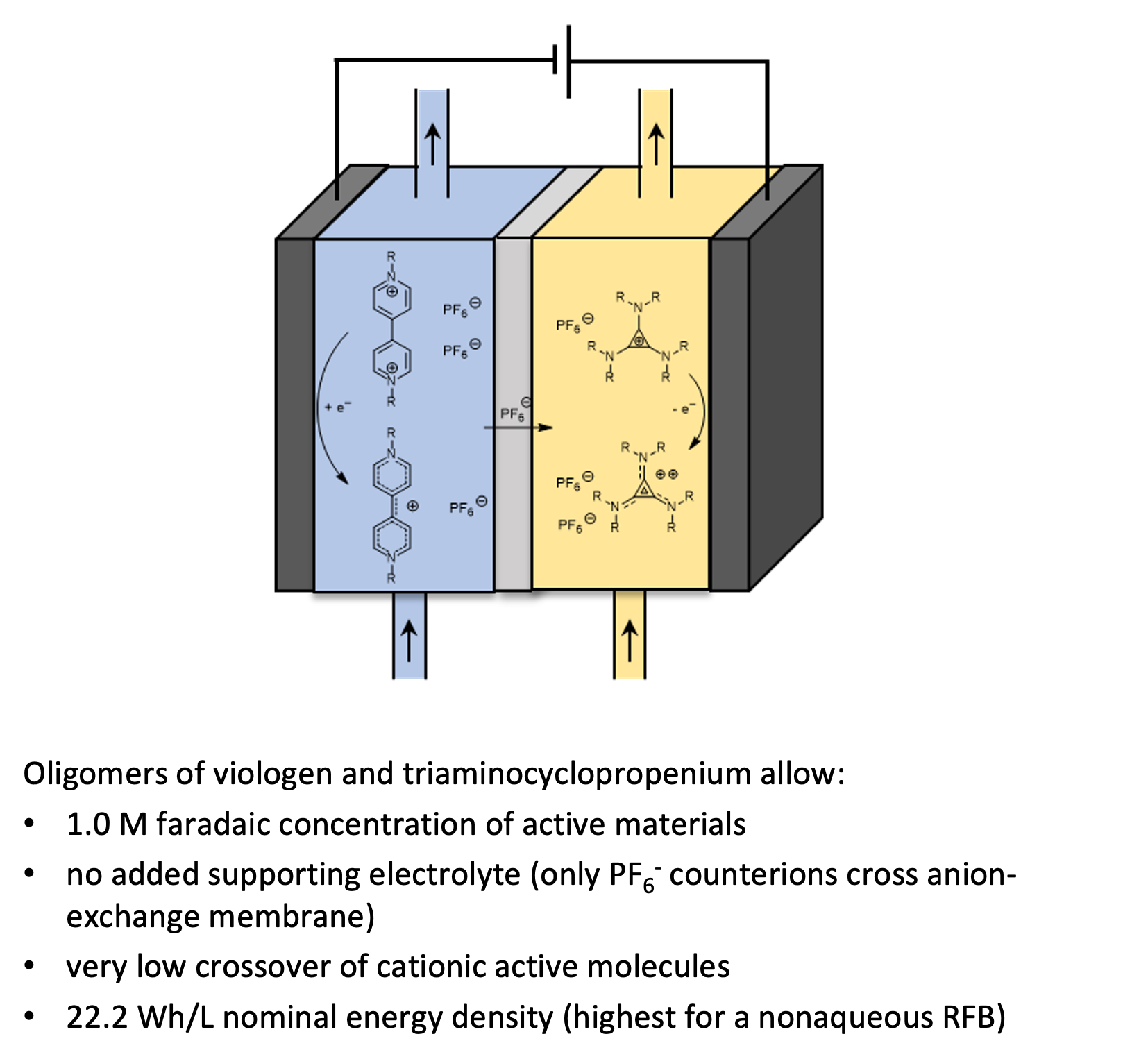
High Energy Density, Asymmetric, Nonaqueous Redox Flow Batteries Without A Supporting Electrolyte
Oligomers of permanently cationic anolyte and catholyte molecules allow the assembly of high energy density nonaqueous redox flow batteries with little crossover of active materials. Read More
-

Multi-chain cation coordination plays key role in increasing glass transition temperature in polymer systems
Free energy calculations show higher thermodynamic stability of multi-chain coordination compared to single-chain coordination in P(EO-MO) resulting in a higher increase in glass transition temperature with addition of salt as compared to PEO Read More
-
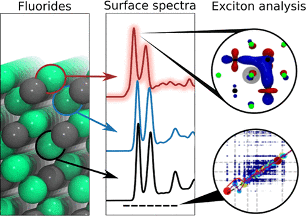
Excitonic Effects in X‐ray Absorption Spectraof Fluoride Salts and Their Surfaces
Advanced calculations of X-ray absorption spectra provide accurate reproduction of experiment, consistent energy alignment and comparative electronic structure interpretation for common fluoride salts often found at electrode interfaces. Read More
-
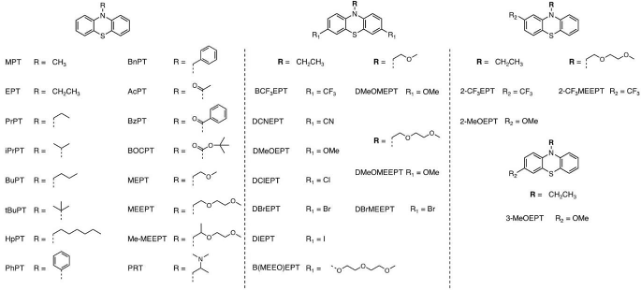
Large Variability and Complexity of Isothermal Solubility for a Series of Redox-Active Phenothiazines
Phenothiazine derivatives present a robust analysis system for experimental solubility determination in all states of charge. The QSPR models were only moderately successful in predicting solubility of an unknown PT compound. Modeling solubility of a fairly complex molecular set with only a few descriptors is difficult. Read More
-
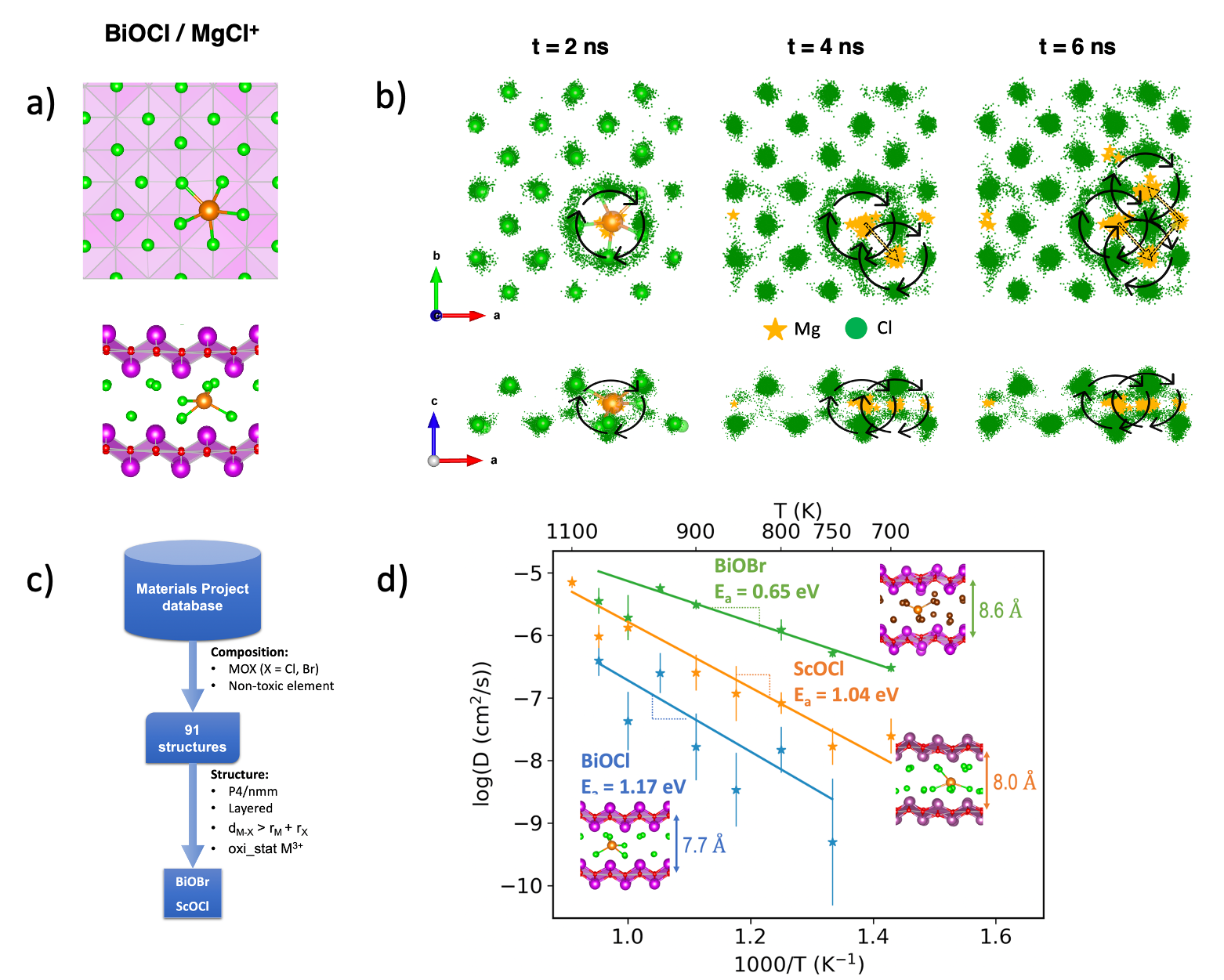
Ionic Dynamics of the Charge Carrier for Mg Batteries
Enabled by the high accuracy and computing efficiency of machine learned interatomic potential trained on-the-fly, we performed dynamic simulation of multivalent ion diffusion in solid materials at nanosecond time scale. Our computations revealed diffusion mechanisms, where cation hopping coordinated by surrounding anions’ synchronous movements in the oxyhalide-based, novel magnesium anode coating materials. Read More
Latest Updates
-
You’re Invited - JCESR and Beyond: Translating the Basic Science of Batteries
Please join us at Argonne National Laboratory on Tuesday, April 4, 2023 for JCESR and Beyond: Translating the Basic Science of Batteries. Registration is now open. This in-person event will celebrate 10 years of research from the Joint Center… Read More
-
A Message from JCESR: In Memory of George Crabtree
It is with heavy hearts that we say goodbye to George Crabtree, a Senior Scientist and Distinguished Fellow at Argonne National Laboratory, and Director of the Joint Center for Energy Storage Research (JCESR), who passed away unexpectedly on January 23. Dr. Read More
-
Cyanopyridines As Extremely Low-Reduction-Potential Anolytes for Nonaqueous Redox Flow Batteries
Discovery of a cyanophenylpyridine derivative with a very low reduction potential and good stability during cycling. Read More
-
Characterizing Redoxmer – Electrode Kinetics Using a SECM-Based Spot Analysis Method
Identified asymmetries in electron transfer (ET) kinetics between the reduction and oxidation of ferrocene-based redoxmers by measuring the ET rate constants (kf/kb) as a function of electrode potential. Read More
-
Benzotriazoles as Low Potential Anolytes for Non-Aqueous Redox Flow Batteries
We developed an easy-to-synthesize benzotriazole-based anolyte with a high energy redox potential (-2.3 V vs Fc/Fc+) and high solubility that demonstrates stable electrochemical cycling performance. Read More

