Flow batteries replace the solid electrodes of conventional batteries with liquid solutions of redox atoms and molecules, enabling large storage capacity for grid-scale applications and long lifetime without the strain of repeated expansion and contraction of solid electrodes during charging and discharging. However, the materials in today’s flow batteries are relatively simple and do not have the flexibility to meet multiple performance metrics simultaneously, such as high energy density, smart responsive behavior and low cost.
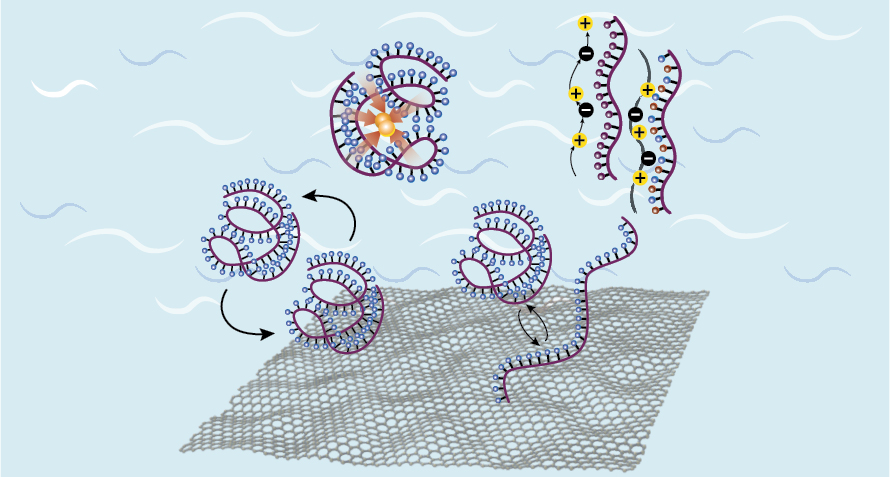
The Flowable Redoxmer Thrust lays the molecular foundation for a new concept in flow batteries introduced in JCESR’s first five years: redox-active polymers, or redoxmers. Redoxmers offer a wealth of design versatility, allowing complex patterns of carbon, hydrogen, oxygen, and nitrogen bonds to translate form into function.
This Thrust expresses JCESR’s mission of building transformative materials from the bottom up, combining atoms and molecules in novel configurations to produce higher operating voltages, higher mobility, longer lifetimes, greater safety, and lower cost in a single structure. There are two focus areas: design of novel redoxmers with unprecedented property combinations, and introduction of smart, responsive, and regenerative behavior.