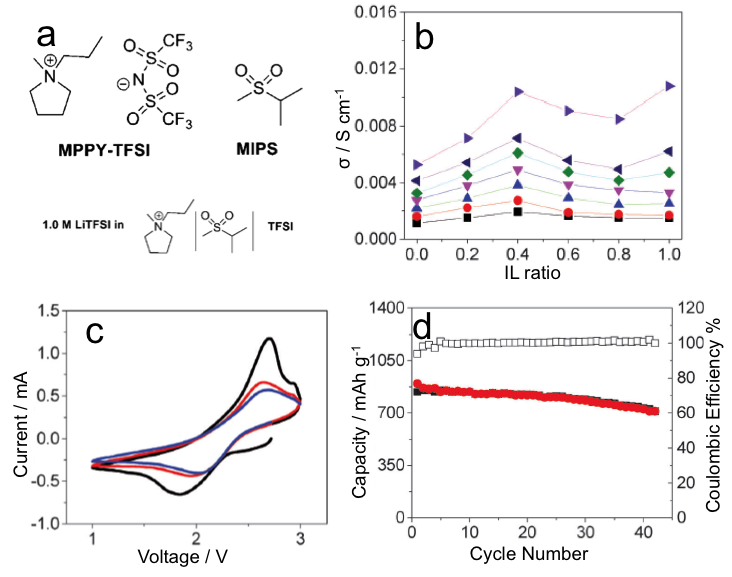
b) ionic conductivity (σ) vs IL ratio
c) CV of C-S cathode in IL/sulfone mixture
d) cycling performance
Scientific Achievement
A strategy of mixing both an ionic liquid and sulfone is applied in Li-S batteries to give synergistic effects of reducing viscosity, increasing ionic conductivity, reducing polysulfide dissolution, and improving safety.
Significance and Impact
The optimum composition ratio for a mixture of IL and sulfone required consideration of both the effects of the suppression of lithium polysulfide formation and reduction of the impedance. Changing the composition ratio of IL and sulfone will change physicochemical properties.
Research Details
- The electrochemical behavior of the Li-S batteries upon using a mesoporous carbon-sulfur (MC-S) composite and various electrolytes was investigated.
- The presence of IL in the sulfone limited lithium polysulfide dissolution, as demonstrated by galvanostatic charge-discharge experiments.
- With the presence of 20% IL in MIPS, the coulombic efficiency decreased from 107 to 100 percent, which suggested a significant decrease in polysulfide dissolution and suppression of the redox shuttle effect.
Work performed at Argonne National Laboratory (JCESR managing partner) and Oak Ridge National Laboratory (JCESR partner) by C. Liao,* BK Guo, XG Sun, and S. Dai, ChemSusChem 2015, 8, 353 – 360.

