Chemical Transformation
-
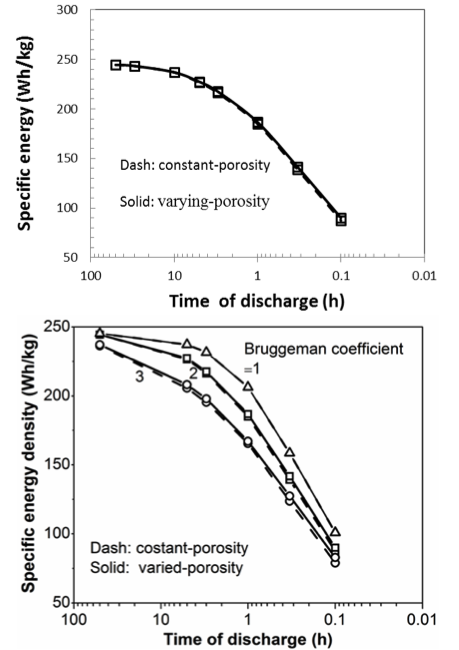
On Graded Electrode Porosity as a Design Tool for Improving the Energy Density of Batteries
This work shows that varying the porosity can only lead to marginal improvements in energy density compared to well-designed constant-porosity electrodes, and suggests that focusing on decreasing the tortuosity would be a better approach. Read More
-
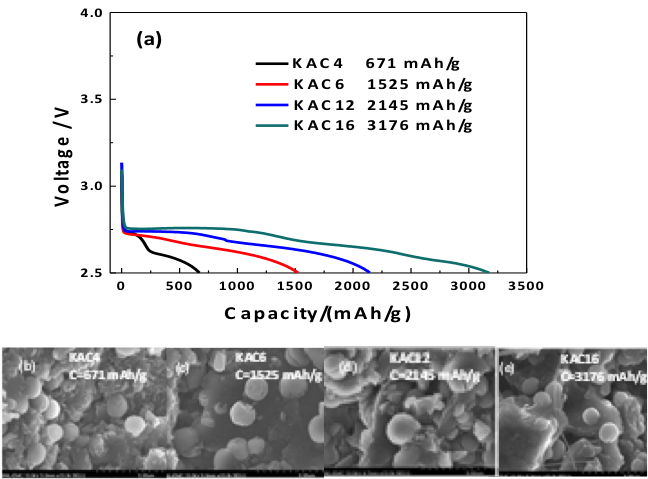
A Functional Impurity for Li-O2 Battery Cathode
Demonstrated that alkali metal can be used as a catalyst Li-O2 cell cathode design and opens the possibility of future optimization of functional K-doping in carbon cathode materials. Read More
-
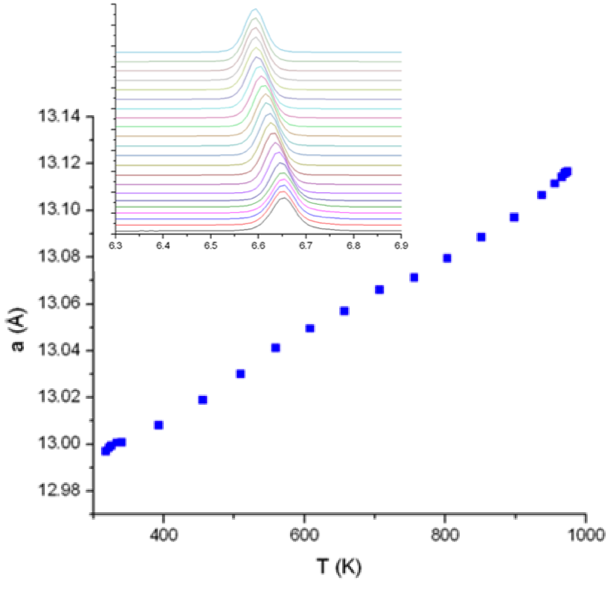
Thermal Expansion in the Garnet-Type Solid Electrolyte (Li7-xAlx/3)La3Zr2O12 as a Function of Al Content
The study identified the lattice expansion lithium lanthanum zirconium oxide (LLZ), which is an important lithium-metal stable solid-state Li-ion diffusion ceramic membrane as a function of Al-content. Read More
-
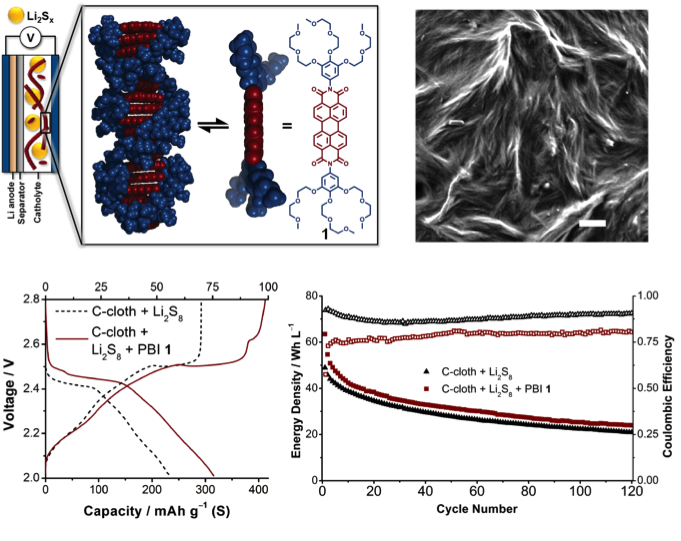
Enhanced Charge Transport in Dissolved Polysulfide Li-S Cells with Supramolecular Redox Mediators
A highly collaborative team of theorists and experimentalists identified a nanostructured redox mediator for soluble polysulfides, synthesized the target compound, electrochemically validate the computations, and demonstrated enhanced current in redox mediated Li-S cells. Read More
-
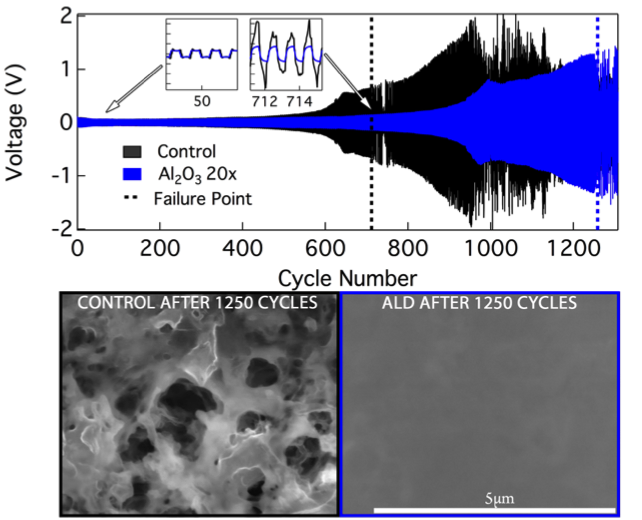
Improved Cycle Life and Stability of Lithium Metal Anodes through Ultrathin ALD Surface Treatments
Li metal foil electrodes treated with ultrathin (~2nm) Al2O3 layers, using Atomic Layer Deposition (ALD), prevent dendrite formation and double the lifetime of the anode before failure under both, galvanostatic deep discharge conditions and cyclic plating/stripping of symmetric Li-Li cells. Read More
-
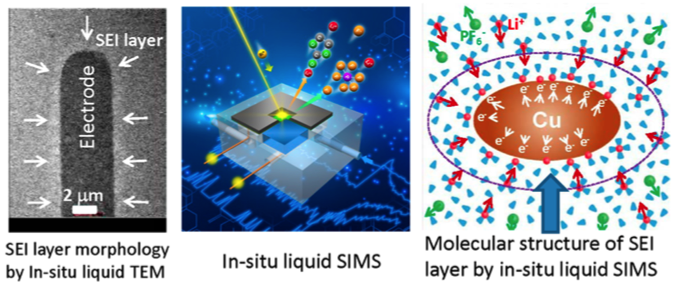
Spectrometric Determination of Molecular Structural Evolution at the Solid Electrolyte Interphase in Lithium-Ion Batteries
Deposition of Li metal on copper electrode leads to condensation of solvent molecules around the electrode. Chemically, this layer of solvent condensate tends to be depleted of the salt anions and have reduced concentration of Li+ ions, essentially leading to formation of a lean electrolyte layer adjacent to the electrode and therefore contributing to the cell overpotential. Read More
-
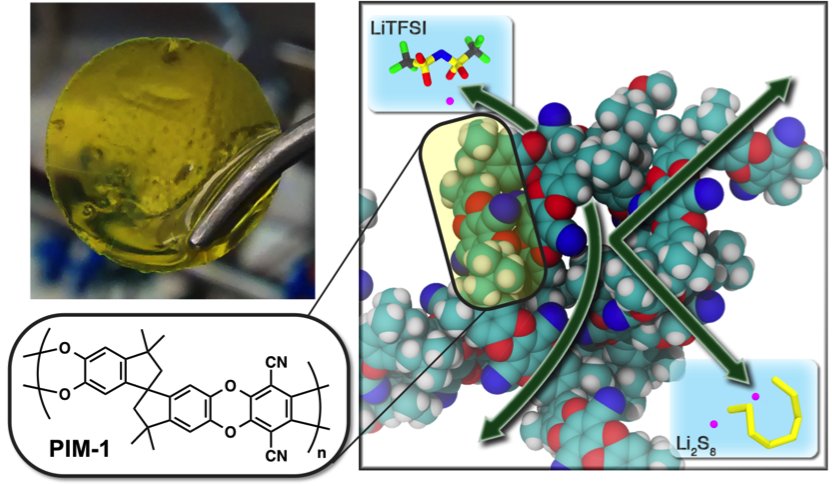
Polysulfide-Blocking Polymer Membrane for Li-S Batteries
olymers of Intrinsic Microporosity are harnessed as an ion-selective membrane, effectively blocking polysulfide crossover due to its microporous molecular-sieving network. Read More
-
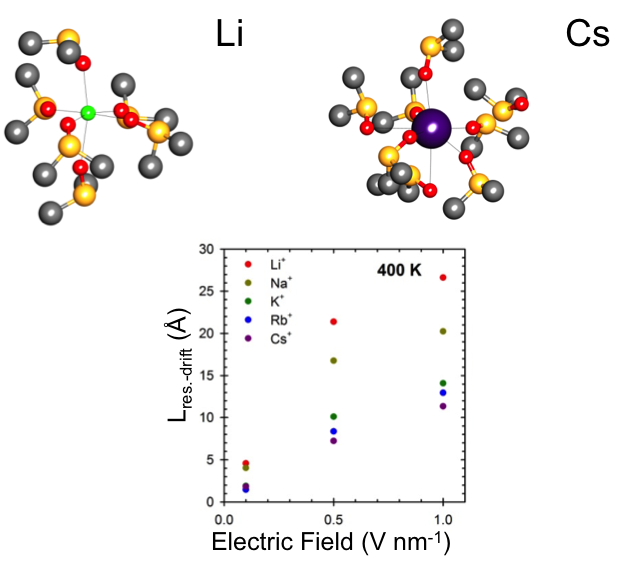
Solvation Structure and Transport Properties of Alkali Cations in Dimethyl Sulfoxide Under Exogenous Static Electric Fields
To predict the behavior of battery electrolyte components, we investigated the role of exogenous electric fields on the solvation structure and dynamics of alkali ions in dimethyl sulfoxide (DMSO). Read More
-
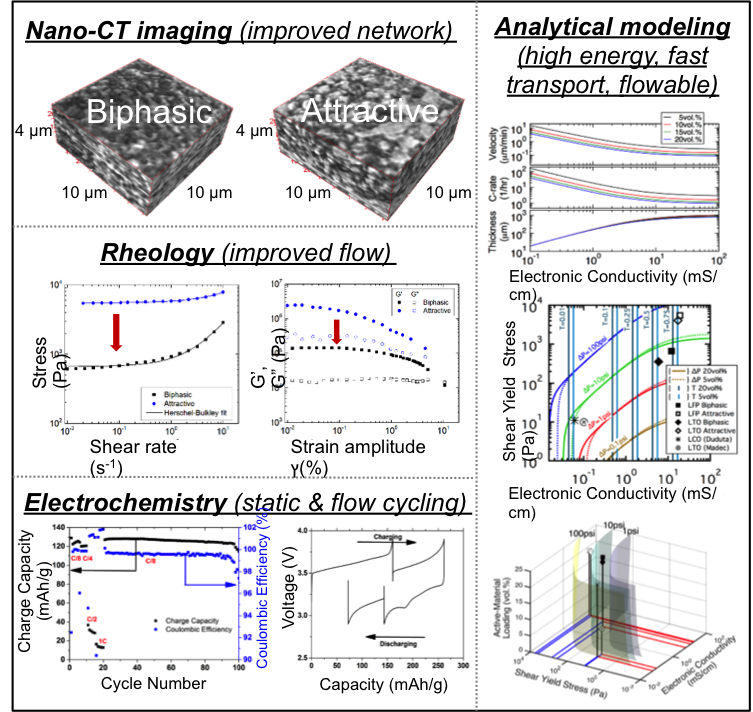
Biphasic Electrode Suspensions for Li-Ion Semi-Solid Flow Cells with High Energy Density, Fast Charge Transport, and Low-Dissipation Flow
We created biphasic electrode suspensions composed of dispersed active particles and uniformly percolated conductive particles, different from the clustered suspensions using traditional suspension preparation procedures. Read More
-
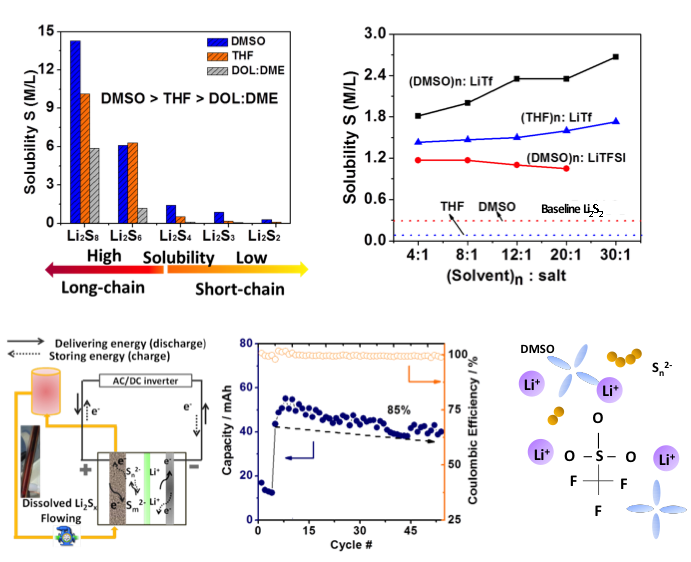
Understanding and Manipulating Solution Chemistry of Polysulfides for Lithium Sulfur Batteries
The factors that govern the solubility of various polysulfides were identified. LiTf with strong ionic association strength increases the solubility of short-chain species significantly. A solution Li-S flow cell was demonstrated using LiTf as additive in the polysulfide catholyte. Read More
Latest Updates
-
You’re Invited - JCESR and Beyond: Translating the Basic Science of Batteries
Please join us at Argonne National Laboratory on Tuesday, April 4, 2023 for JCESR and Beyond: Translating the Basic Science of Batteries. Registration is now open. This in-person event will celebrate 10 years of research from the Joint Center… Read More
-
A Message from JCESR: In Memory of George Crabtree
It is with heavy hearts that we say goodbye to George Crabtree, a Senior Scientist and Distinguished Fellow at Argonne National Laboratory, and Director of the Joint Center for Energy Storage Research (JCESR), who passed away unexpectedly on January 23. Dr. Read More
-
Cyanopyridines As Extremely Low-Reduction-Potential Anolytes for Nonaqueous Redox Flow Batteries
Discovery of a cyanophenylpyridine derivative with a very low reduction potential and good stability during cycling. Read More
-
Characterizing Redoxmer – Electrode Kinetics Using a SECM-Based Spot Analysis Method
Identified asymmetries in electron transfer (ET) kinetics between the reduction and oxidation of ferrocene-based redoxmers by measuring the ET rate constants (kf/kb) as a function of electrode potential. Read More
-
Benzotriazoles as Low Potential Anolytes for Non-Aqueous Redox Flow Batteries
We developed an easy-to-synthesize benzotriazole-based anolyte with a high energy redox potential (-2.3 V vs Fc/Fc+) and high solubility that demonstrates stable electrochemical cycling performance. Read More

