Chemical Transformation
-
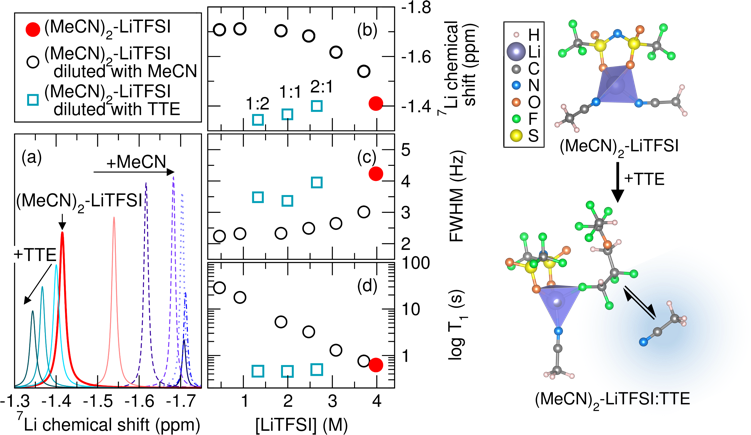
The Effect of Hydrofluoroether Addition on S8 Reduction and the Li+ Solvation Structure in the Solvate Electrolyte
The hydrofluoroether, TTE, competes with MeCN coordination to Li+ in the solvate electrolyte resulting in a higher free MeCN content as TTE is added. The content of free MeCN affects S8 reduction kinetics likely through facilitation of polysulfide formation and enhanced local solvation effects. Read More
-
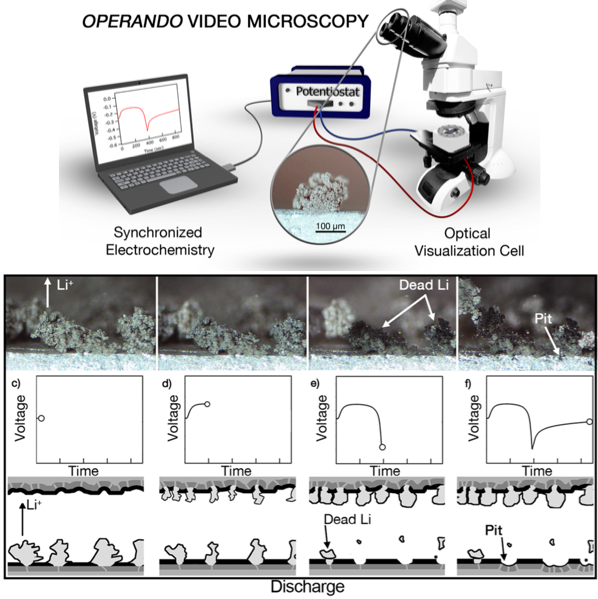
Dendrites and Pits: Untangling the Complex Behavior of Lithium Metal Anodes through Operando Video Microscopy
A mechanistic understanding of the complex cycling behavior of Li metal anodes has been gained by combining operando video microscopy with continuum-scale modeling of Li/Li symmetric cells. Read More
-

Sparingly Solvating Electrolytes for High Energy Density Lithium-Sulfur Batteries
As JCESR scientists work to develop lighter and less expensive chemistries than those used in current lithium-ion batteries, lithium-sulfur shows tremendous promise. This perspective presents an alternate approach that could move us closer to long-lived, high energy density lithium-sulfur batteries. Read More
-
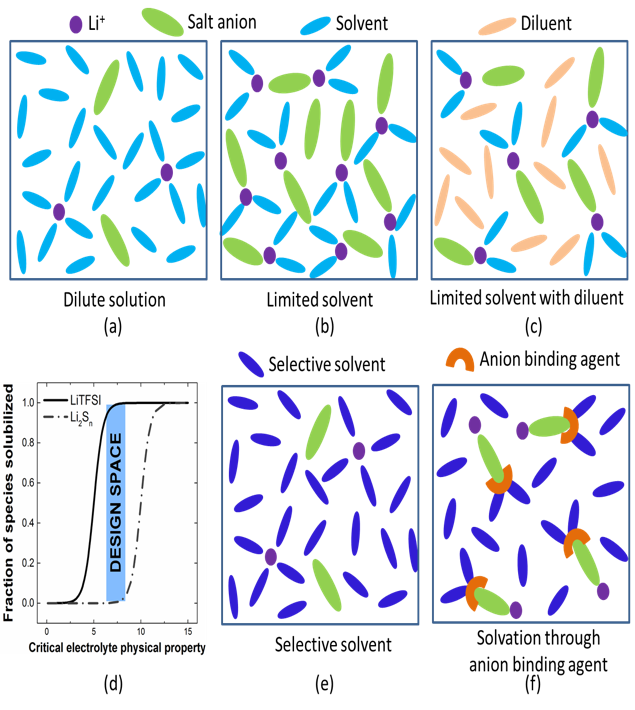
Sparingly Solvating Electrolytes for High Energy Density Lithium-Sulfur Batteries
This work presents the promising new concepts of using sparingly solvating electrolyte to enable Li-S battery operation at lean electrolyte condition, as well as the design rules for discovering new electrolyte systems. Read More
-
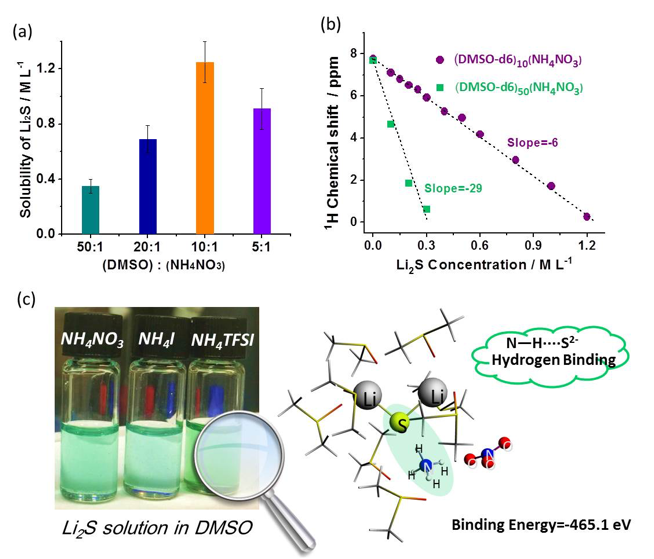
Ammonium Additives to Dissolve Li2S through Hydrogen Binding for High Energy Li-S Batteries
Ammonium salts are demonstrated as effective additives to promote the dissolution of Li2S (up to concentrations of 1.25 M) in DMSO solvent at room temperature through hydrogen binding between N-H groups and S2- anions. Read More
-
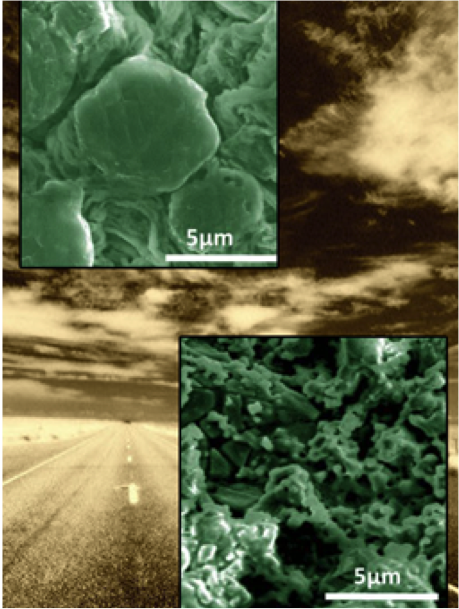
Effect of the Anion Activity on the Stability of Li Metal Anodes in Lithium-Sulfur Batteries
Discovered why the salt LiTFSI -- when added to the electrolyte of a Li-S battery -- allows the battery to hold a charge much longer than other salts Read More
-
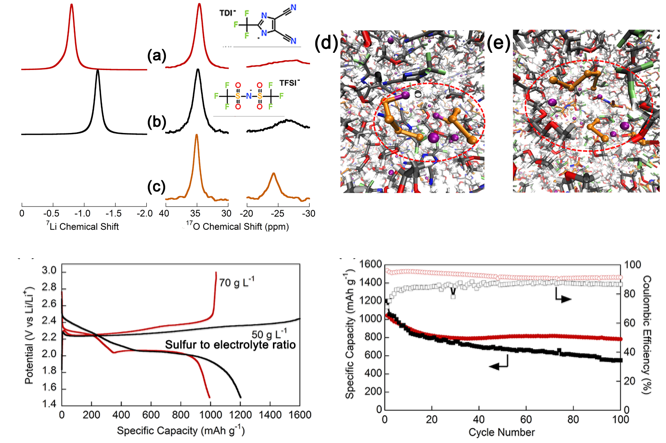
Restricting the Solubility of Polysulfides in Li-S Batteries Via Electrolyte Salt Selection
Lithium 2-trifluoromethyl-4,5-dicyanoimidazole (LiTDI) as a supporting salt in electrolytes suppresses the maximum solubility of Li2S8 by forming a Li4S8 dimer rather than the Li2S3 and Li2S5 observed in a LiTFSI electrolyte, which enables a cell with a high sulfur loading (3 mg-S cm-2) to deliver a 1.67 mAh cm-2 areal capacity after 300 stable cycles at a high current density (2.4 mA cm-2). Read More
-
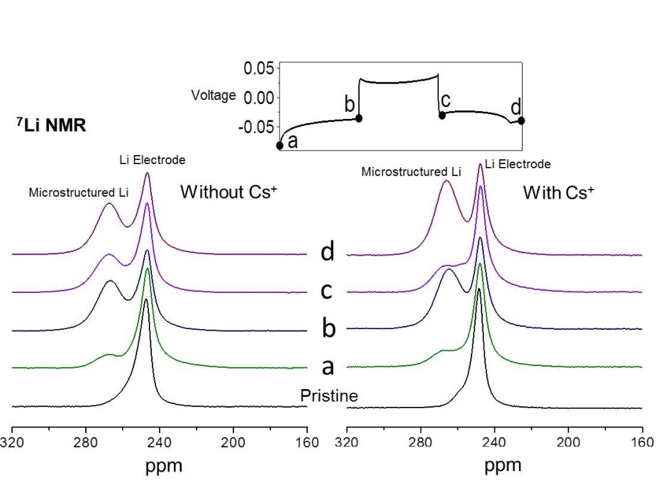
In situ 7Li and 133Cs NMR Investigations of the Role of Cs+ Additive in Lithium-Metal Deposition Processes
Insights are obtained into the mechanisms of adding Cs+ to protect the Li-metal electrode during battery cycling. Read More
-

Redox Mediators that Promote Three-Dimensional Growth of Li2S on Carbon Current Collectors in Lithium-Sulfur Batteries
Developed, from computation and experiment, redox mediators that allow 3-D growth of Li2S on carbon current collectors for greater capacity utilization in Li-S batteries Read More
-
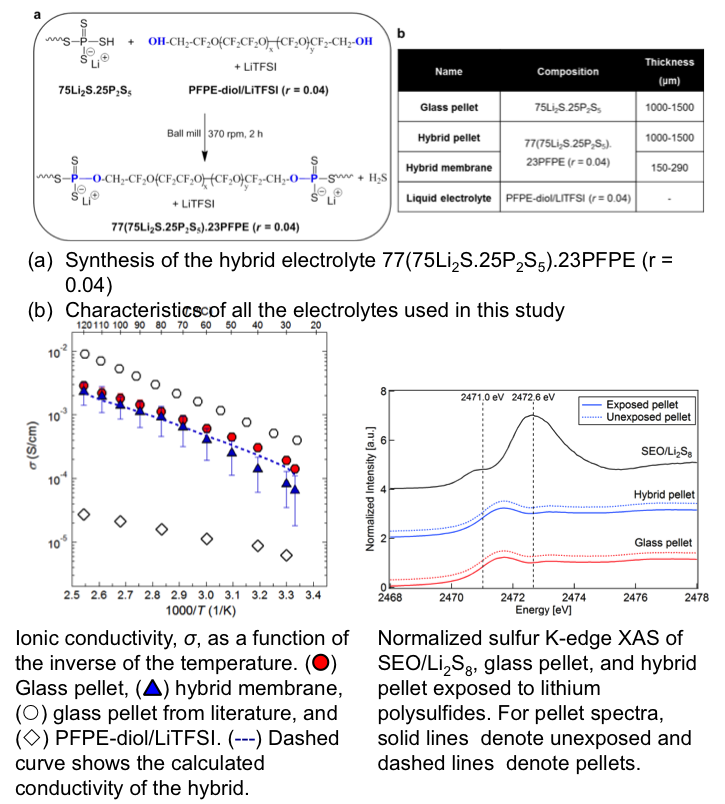
Compliant Glass–Polymer Hybrid Single Ion-Conducting Electrolytes for Lithium Batteries
We have successfully developed non-flammable hybrid single-ion-conducting electrolytes comprising inorganic sulfide glass particles covalently bonded to a perfluoropolyether polymer. These electrolytes present high transference numbers, unprecedented ionic conductivities at room temperature, excellent electrochemical stability, and limit the dissolution of lithium polysulfides. Read More
Latest Updates
-
You’re Invited - JCESR and Beyond: Translating the Basic Science of Batteries
Please join us at Argonne National Laboratory on Tuesday, April 4, 2023 for JCESR and Beyond: Translating the Basic Science of Batteries. Registration is now open. This in-person event will celebrate 10 years of research from the Joint Center… Read More
-
A Message from JCESR: In Memory of George Crabtree
It is with heavy hearts that we say goodbye to George Crabtree, a Senior Scientist and Distinguished Fellow at Argonne National Laboratory, and Director of the Joint Center for Energy Storage Research (JCESR), who passed away unexpectedly on January 23. Dr. Read More
-
Cyanopyridines As Extremely Low-Reduction-Potential Anolytes for Nonaqueous Redox Flow Batteries
Discovery of a cyanophenylpyridine derivative with a very low reduction potential and good stability during cycling. Read More
-
Characterizing Redoxmer – Electrode Kinetics Using a SECM-Based Spot Analysis Method
Identified asymmetries in electron transfer (ET) kinetics between the reduction and oxidation of ferrocene-based redoxmers by measuring the ET rate constants (kf/kb) as a function of electrode potential. Read More
-
Benzotriazoles as Low Potential Anolytes for Non-Aqueous Redox Flow Batteries
We developed an easy-to-synthesize benzotriazole-based anolyte with a high energy redox potential (-2.3 V vs Fc/Fc+) and high solubility that demonstrates stable electrochemical cycling performance. Read More

