NOBF4 (strong oxidant, not suitable for many compositions, ~$600/mol)
Et3OBF4 (mild, but still causes adatom desorption for some sensitive compositions, ~$800/mol)
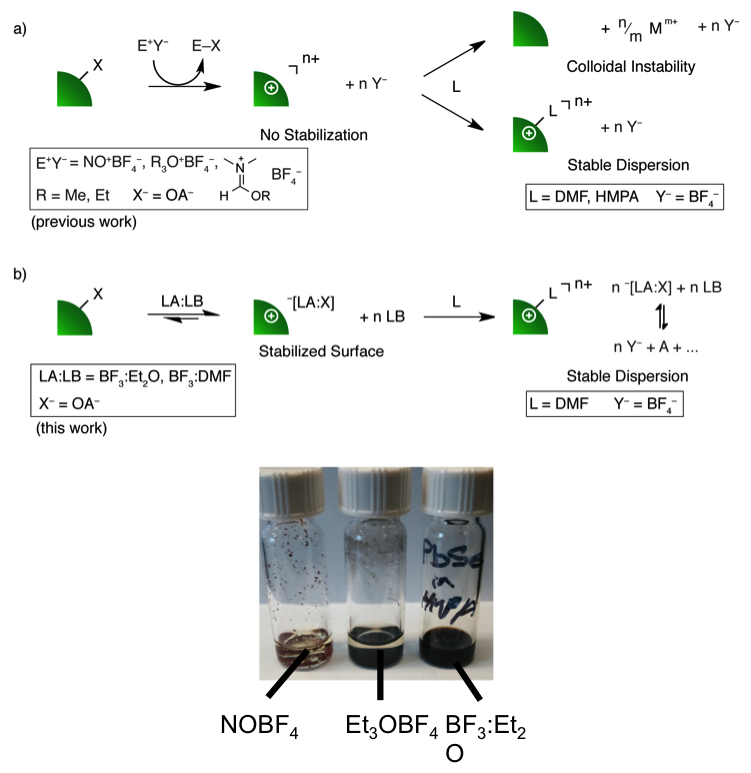
New ligand-stripping chemistry developed within JCESR:
BF3:Et2O (mildest stripping reagent to date, <$18/mol)
Scientific Achievement
Developed a class of ligand-stripping reagents that stabilize the nanocrystal (NC) surface during the stripping reaction, leading to improved dispersibility.
Significance and Impact
- NCs show promise as energy storage and conversion materials, but they are often synthesized with surface-bound organic ligands that improve dispersibility while blocking charge transport. Existing methods for removing these ligands are expensive and lead to poor dispersibility for some materials.
- Ligand stripping under equilibrium control can be used to generate materials that are impossible to produce by existing methods at low cost.
Research Details
1H-DOSY showed that ligand-stripping proceeds through the formation of a Lewis acid-base adduct, which stabilizes the NC surface. ESI-MS showed that this adduct breaks down to form neutral species and non-coordinating counter-ions. The resulting NC dispersions were readily deposited as thin films or polymer-NC hybrids.
Work performed at Lawrence Berkeley National Laboratory (JCESR partner) by S. E. Doris, J. J. Lynch, C. Li, A. W. Wills, J. J. Urban and B. A. Helms . J. Am. Chem. Soc. 2014, 136 (44), 15702–15710.
DOI: 10.1021/ja508675t

