Multivalent Intercalation
-
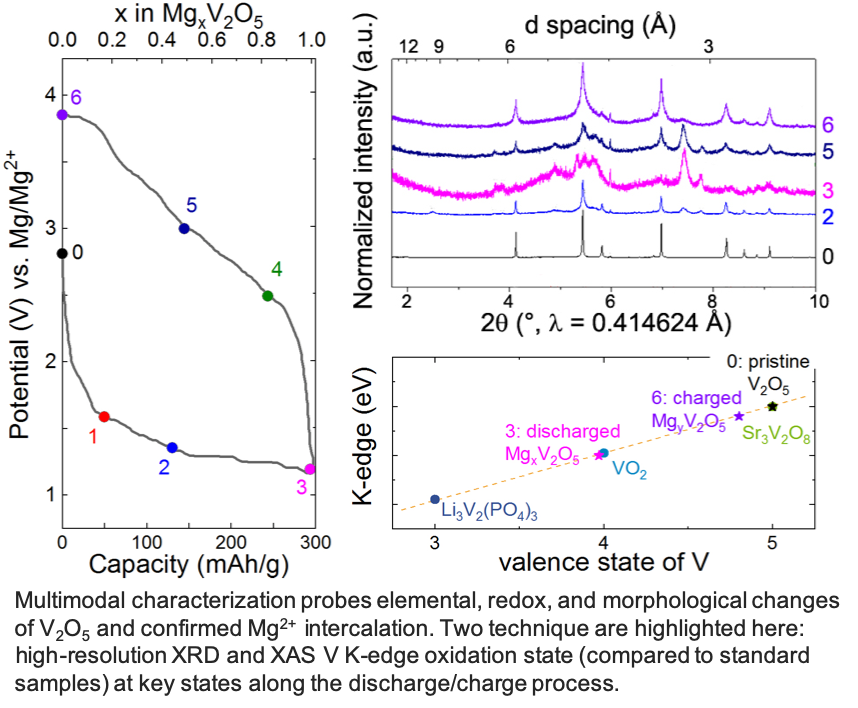
Intercalation of Magnesium into a Layered V2O5
Layered a-V2O5 reversibly intercalates 1 Mg2+ per unit formula to achieve 280 mAh/g at 110°C in a chemically and anodically stable ionic liquid electrolyte. Read More
-
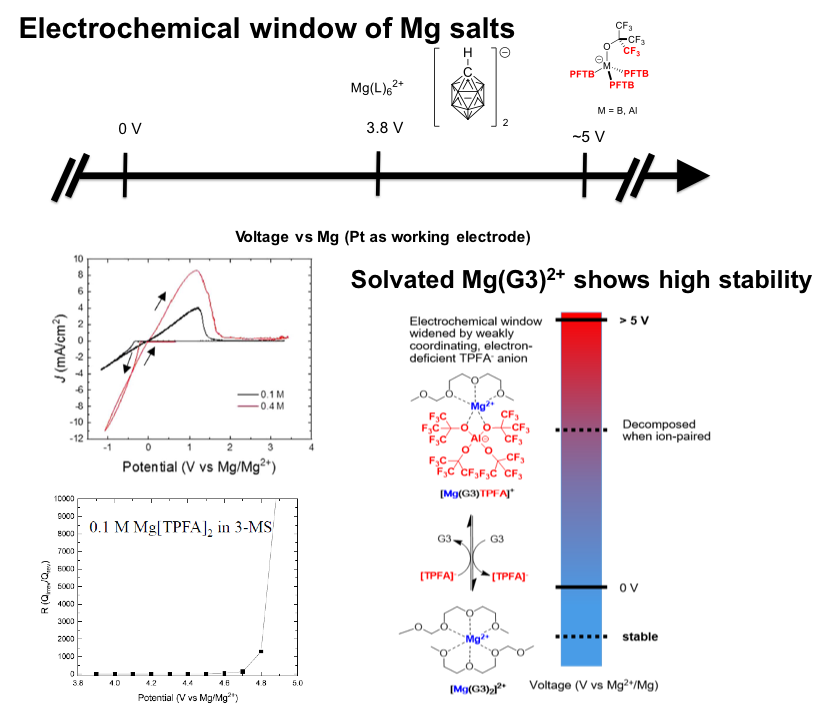
Widening Electrochemical Window of Mg Salt by Weakly Coordinating Perfluoroalkoxyaluminate Anion for Mg Battery Electrolyte
Mg[Al{OC(CF3)3}4]2 (Mg[TPFA]2 ) with perfluorinated, e- deficient anions ([TPFA]−) possesses high thermodynamic oxidative stability with high coulombic efficiency for Mg plating/stripping. Read More
-
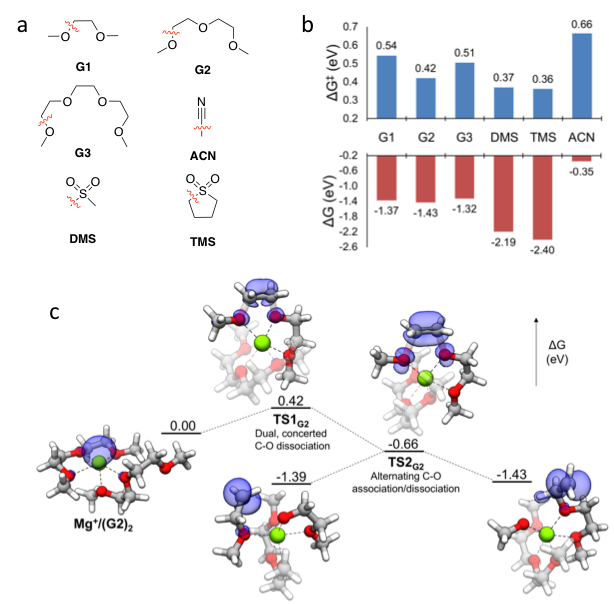
First-principles Exploration of Stability and Associated Decomposition Mechanisms of Solvents in Mg Energy Storage
Obtained a deeper understanding of the chemical and electrochemical stability governing the suitability of several organic solvents commonly considered for Mg battery electrolytes. Read More
-
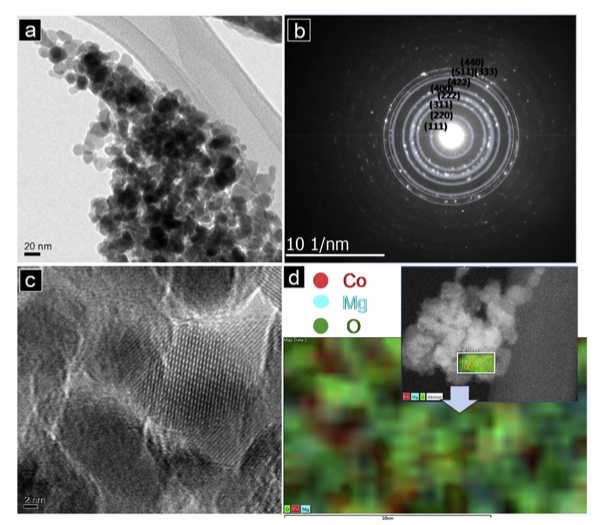
Direct Observation of MgO Formation at the Cathode-Electrolyte Interface of a Spinel MgCo2O4 Cathode upon Electrochemical Mg+2 Removal and Insertion
We have been exploring the versatility and mechanism of Mg insertion into several transition metal spinel compounds and correlating the results with electron count. In this publication we established that the MgCo2O4 works by a conversion mechanism – MgO extraction and precipitation as the primary electrochemical activity with loss of oxide and reduction of cobalt. System characterization was complicated by the observed solid solution between MgCo2O4 – Co3O4 but overcome by utilizing a variety of techniques to fully characterize the materials synthesized. Read More
-
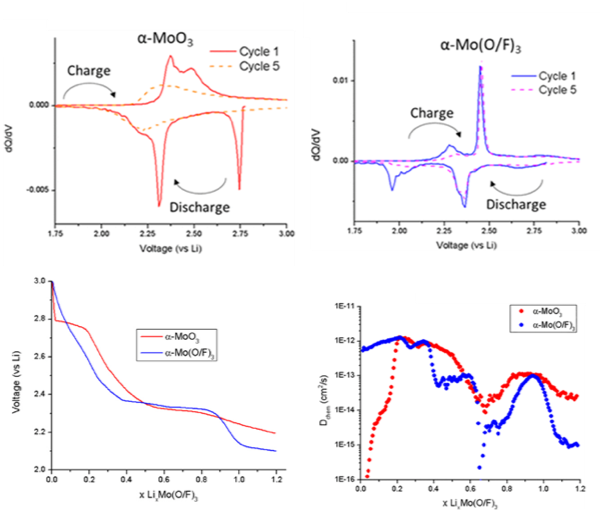
Fluoride Doping Enhances Lithium Diffusivity in Layered Molybdenum Oxide
By fluoride doping MoO3 to MoO2.8F0.2 the reversibility of lithium intercalation could be greatly increased and a sluggish intercalation step could be bypassed. Read More
-
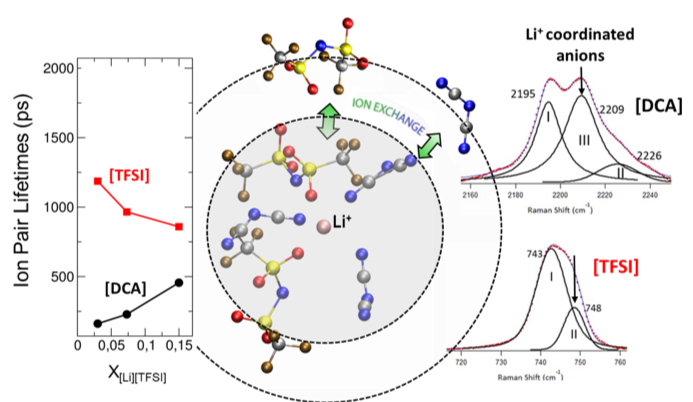
Solvation Structure and Dynamics of Li+ in Ternary Ionic Liquid-Lithium Salt Electrolytes
The local structure of Li+ in this eutectic is found to be heterogenous and preferentially solvated by [DCA], which is related to the transport properties. Read More
-
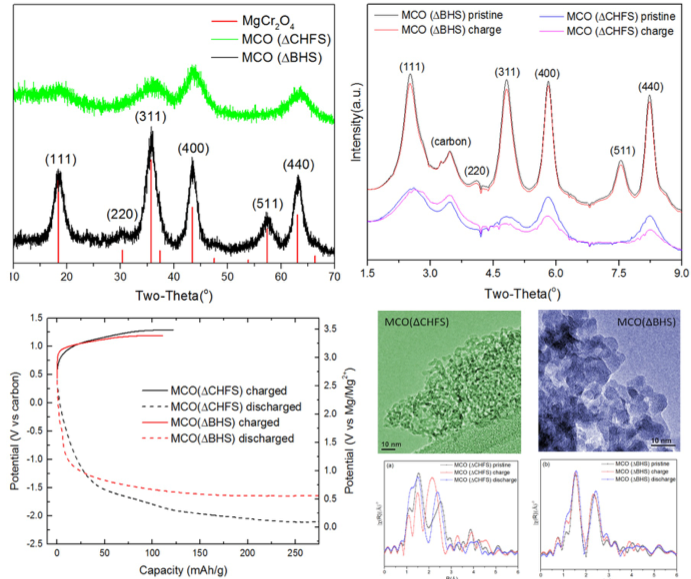
Tailoring the Electrochemical Activity of Magnesium Chromium Oxide Towards Mg Batteries Through Control of Size and Crystal Structure
Reversible magnesium removal from MgCr2O4 was induced by nanosizing and introducing significant structural defects to reduce diffusion the distance and overcome the activation energy barrier. Read More
-
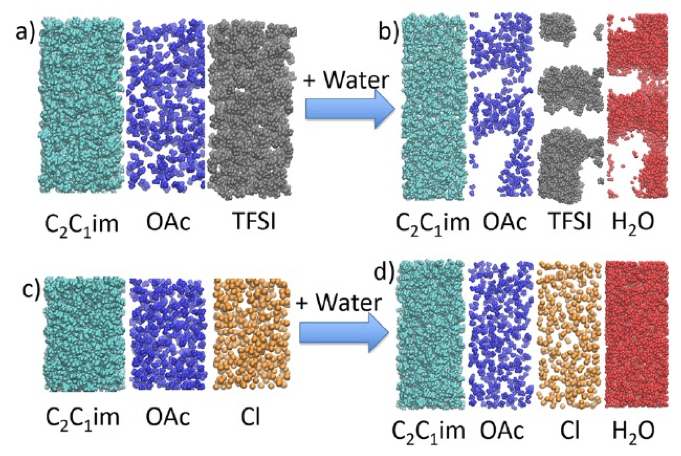
Simulation and Measurement of Water-induced Liquid-liquid Phase Separation of Imidazolium Ionic Liquid Mixtures
Computationally predicted liquid-liquid phase equilibrium confirmed by experimental measurements. Read More
-
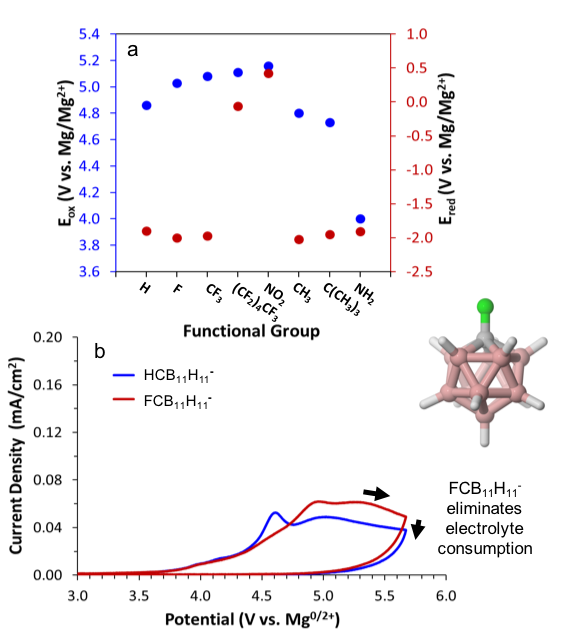
Rational Design of Stable Multivalent Electrolytes and Interphases
Demonstrated a strategy for expanding the electrochemical stability window for magnesium batteries through predictive anion design while discovering new mechanisms of interphase formation. Read More
-
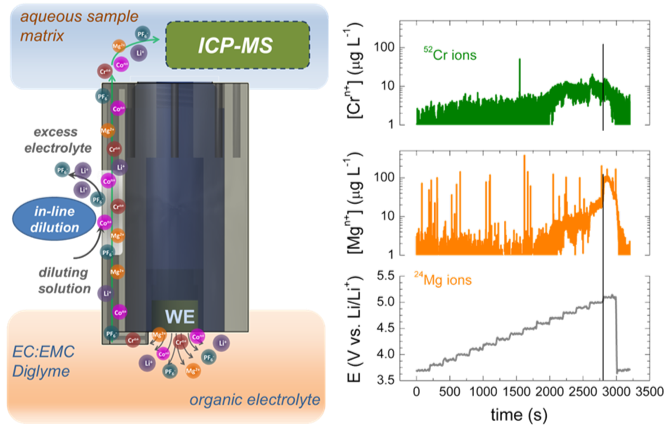
Real-Time Monitoring of Cation Dissolution/Deintercalation Kinetics from Transition-Metal Oxides in Organic Environments
A new method that allows in situ, real-time monitoring of dissolution rates of transition metal cations from oxide cathode hosts, as well as monitoring of deintercalation of the working cation (Li, Mg, Zn, etc.) simultaneously with electrochemical cycling. Read More
Latest Updates
-
You’re Invited - JCESR and Beyond: Translating the Basic Science of Batteries
Please join us at Argonne National Laboratory on Tuesday, April 4, 2023 for JCESR and Beyond: Translating the Basic Science of Batteries. Registration is now open. This in-person event will celebrate 10 years of research from the Joint Center… Read More
-
A Message from JCESR: In Memory of George Crabtree
It is with heavy hearts that we say goodbye to George Crabtree, a Senior Scientist and Distinguished Fellow at Argonne National Laboratory, and Director of the Joint Center for Energy Storage Research (JCESR), who passed away unexpectedly on January 23. Dr. Read More
-
Cyanopyridines As Extremely Low-Reduction-Potential Anolytes for Nonaqueous Redox Flow Batteries
Discovery of a cyanophenylpyridine derivative with a very low reduction potential and good stability during cycling. Read More
-
Characterizing Redoxmer – Electrode Kinetics Using a SECM-Based Spot Analysis Method
Identified asymmetries in electron transfer (ET) kinetics between the reduction and oxidation of ferrocene-based redoxmers by measuring the ET rate constants (kf/kb) as a function of electrode potential. Read More
-
Benzotriazoles as Low Potential Anolytes for Non-Aqueous Redox Flow Batteries
We developed an easy-to-synthesize benzotriazole-based anolyte with a high energy redox potential (-2.3 V vs Fc/Fc+) and high solubility that demonstrates stable electrochemical cycling performance. Read More

