In the past five years, our research focused on three legacies:
- A library of the fundamental science of the materials and phenomena of energy storage at atomic and molecular levels
- A transportation prototype and a grid prototype that significantly reduce cost/kWh and increase energy density
- A new paradigm for battery research that integrates discovery science, battery design, research prototyping, and manufacturing collaboration in a single highly interactive organization
Fundamental science outcomes include innovative tools (see sidebar) and frontier science advances:
- Prediction and validation of fast solid-state mobility of Mg2+
- Discovery of a primary failure mode of multivalent electrolytes: the high reactivity of the intermediate charge state Mg+ cleaves multivalent electrolyte molecules
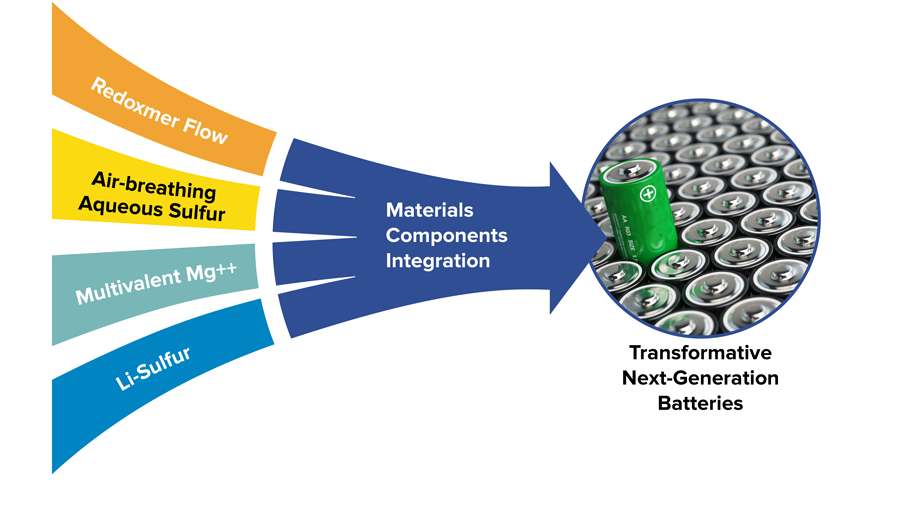
- Introduction and development of redoxmers, a new concept for flow batteries
- Development of a machine learning approach to discover a redoxmer with 1,000 times the stability
- Introduction and development of molecular separation based on charge and size in polymer membranes
JCESR science advances led to three startups based on JCESR intellectual property:
- Blue Current, for solid state electrolytes
- Sepion, for size-selective polymer membranes
- Form Energy, for commercializing air-breathing aqueous sulfur batteries for inexpensive, long-duration energy storage
Tomorrow’s Legacy
Today’s batteries meet only some of their targeted performance metrics. Meeting all of the targeted performance metrics for a given application requires new materials with transformative behavior, such as simultaneously achieving high reactivity, high mobility and high stability in electrodes, or high selectivity and stability at low cost in membranes. Such transformative materials are not readily available in nature. They must be designed based on an intimate knowledge of the atomic and molecular origins of the targeted behavior.
Imagine the impact on battery science and technology of a unified description of ion solvation in liquids and solids at the atomic level, a template for designing redox active multimers (redoxmers) that allows adjusting reactivity, solubility, and stability independently, or a full understanding of the roles of defects in crystals and heterogeneity at interfaces on overall materials behavior.
Breakthroughs in these areas would permanently alter the face of battery and electrochemical science and would lead to a blossoming of new materials with unique electroactive function. Most importantly, these three fundamental breakthroughs would usher in a new era of constructionist “bottom up” atom-by-atom and molecule-by-molecule materials design, with each atom or molecule playing a prescribed role in producing a targeted overall behavior.