Redox Flow
-
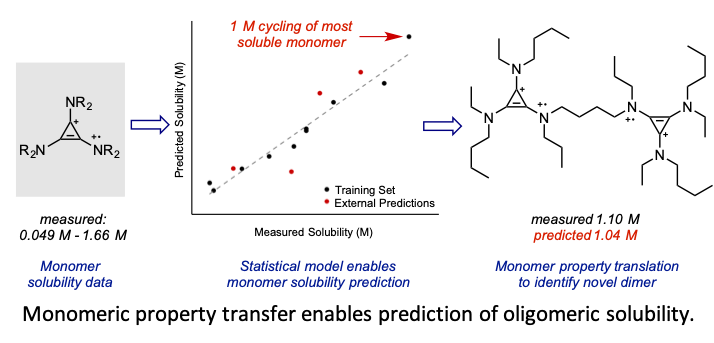
Developing a Predictive Solubility Model for Monomeric and Oligomeric Cyclopropenium-Based Flow Battery Catholytes
Two important field-wide goals are met through this study: the development of a statistical model for the solubility of conformationally flexible molecules in acetonitrile and the operation of a high concentration (1 M) nonaqueous organic symmetric flow cell. Read More
-
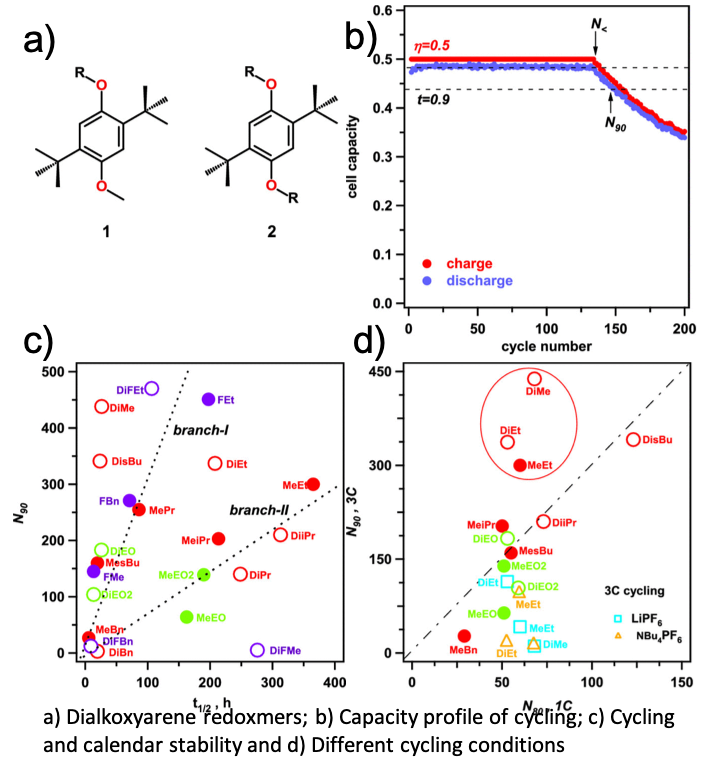
On Transferability of Performance Metrics for Redox-active Molecules
It is shown that two commonly used performance metrics for charged redoxmers in flow batteries, viz. their cycling and calendar lifetimes, generally do not correlate with each other. It is also demonstrated that the cycling stability is extremely sensitive to experimental detail; unless the conditions are tightly controlled, this metric is unsuitable as a guide for materials development. Read More
-

Stability and Microheterogeneity in Concentrated Nonaqueous Electrolyte Solutions
We compared the molecular dynamics of two different but structurally-related electrolytes in highly concentrated solutions. One catholyte is compact and symmetrical; the other is bulkier and less symmetrical. Incoherent elastic and quasi-elastic neutron scattering measurements showed significant differences in crystallization, supercooling, and sudden microheterogeneity. Read More
-
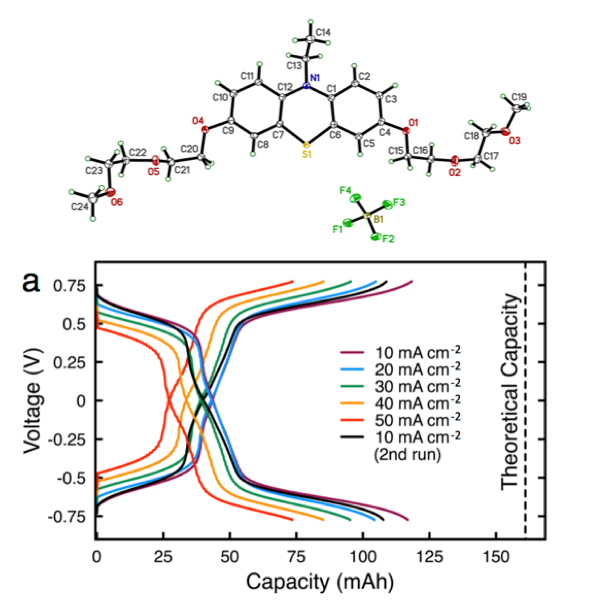
Enabling High Concentration Redox Electrolytes for Use in Nonaqueous Redox Flow Batteries
Building on prior collaborative JCESR research on iteratively advancing phenothiazines as charge storage materials, we developed a new class of phenothiazine derivatives with high equivalent charge concentration by increasing solubility and stability of multiple electron transfer events. Read More
-
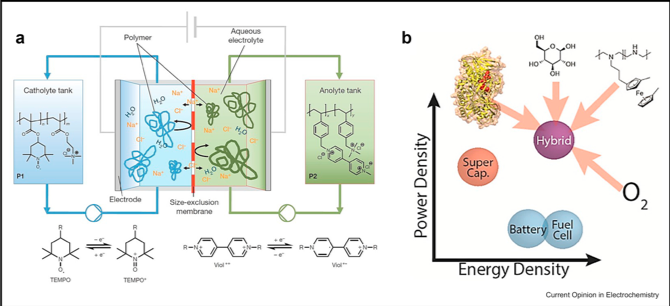
Redox Polymers in Electrochemical Systems: From Methods of Mediation to Energy Storage
Redox polymers were designed for mediation in sensors in the 1980s, but it was not until the last five years that researchers realized that they could play a critical role in energy storage, including redox flow batteries and supercapacitors. Read More
-
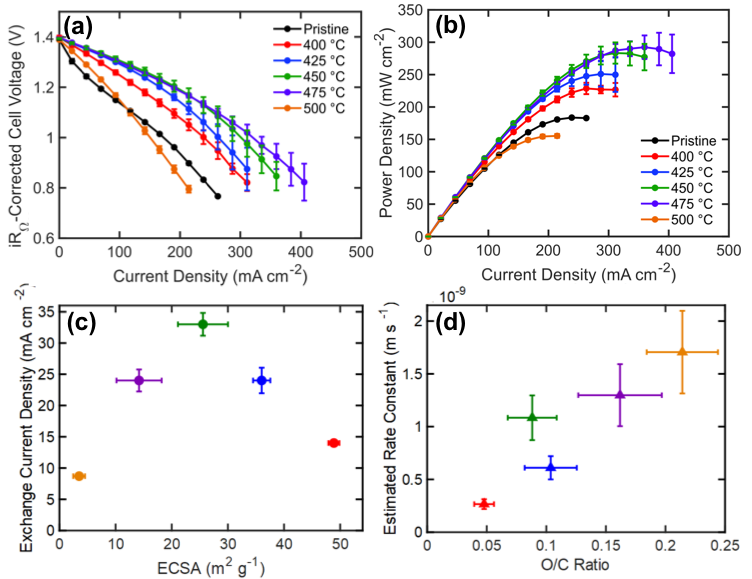
Elucidating the Nuanced Effects of Thermal Pretreatment on Carbon Paper Electrodes for Vanadium Redox Flow Batteries
The chemical and physical properties of carbon paper electrodes treated at temperatures between 400-500 °C for 30 hours were quantified, and the subsequent impact of these properties on the performance of vanadium redox flow batteries explained. We find that the oxygen content and electrochemically active surface area, two parameters that govern the kinetic performance of the vanadium flow battery, follow opposing temperature trends; thus, optimal performance is achieved at a balance of these properties rather than a maximization of each. Read More
-
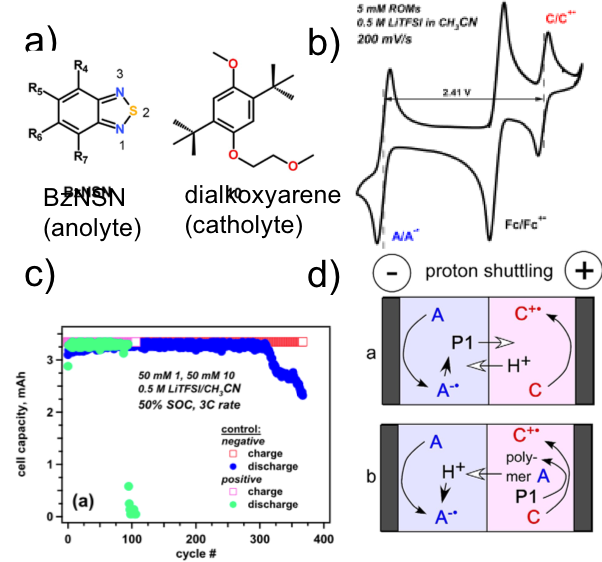
Comparing Calendar and Cycle Life Stability of Redox Active Organic Molecules for Nonaqueous Redox Flow Batteries
Cycle and calendar lives are chemical stability metrics that reflect two regimes of redox flow battery use: (i) continuous charge/discharge cycling and (ii) long-term charge storage in external tanks. Here we examine how these two performance metrics are related to each other for negative charge carriers (anolytes) in nonaqueous flow cells. Surprisingly, little correlation was found between these two practically important metrics. Read More
-
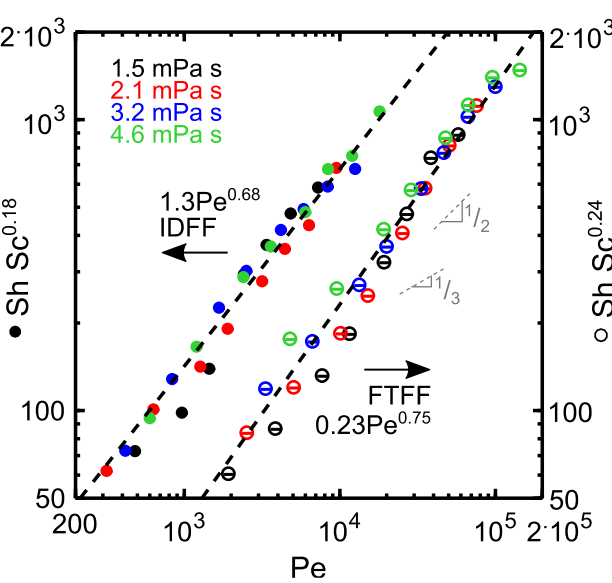
Quantifying the Impact of Viscosity on Mass-transfer Coefficients in Redox Flow Batteries
A methodology for and the results from quantification of the mass-transfer coefficient is demonstrated in the single-electrolyte configuration of JCESR’s Gen-2 cell using a model redox-active electrolyte (RAE) with a tunable viscosity. Read More
-
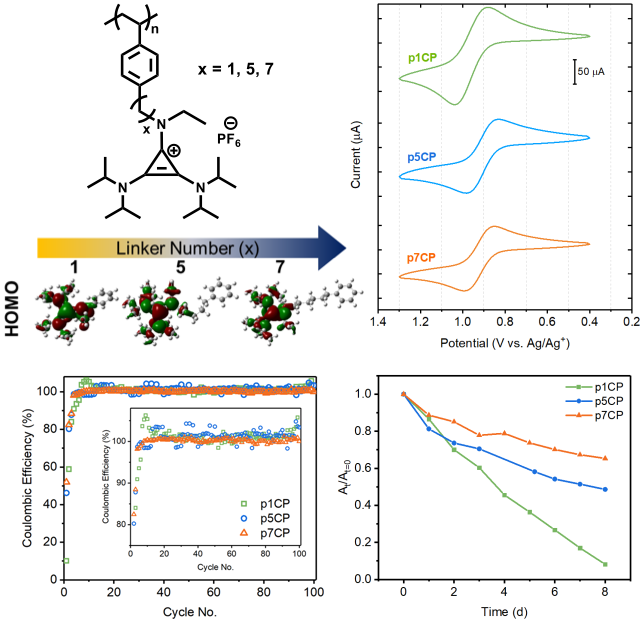
Effect of the Backbone Tether on the Electrochemical Properties of Soluble Cyclopropenium Redox-Active Polymers
A series of cyclopropenium polymers were synthesized and evaluated as high potential active material for size-exclusion flow batteries. Read More
-
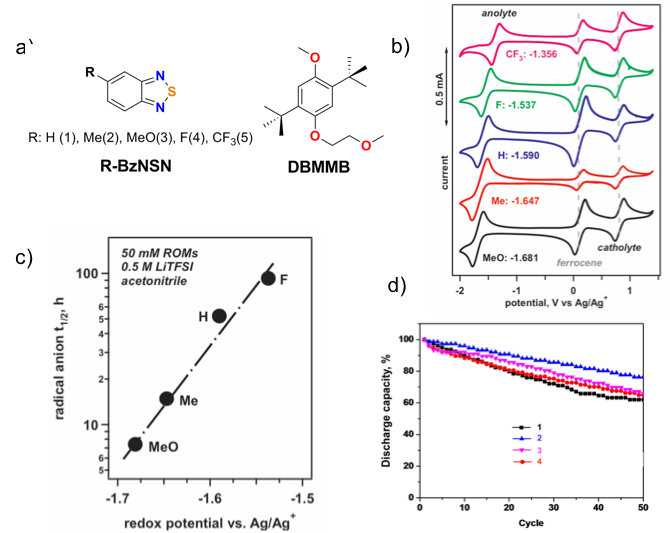
Substituted Thiadiazoles as Energy-rich Anolytes for Nonaqueous Redox Flow Cells
2,1,3-benzothiadiazole (BzNSN) base molecules were derivatized with various electronic groups to achieve the optimal properties as the anolytes in nonaqueous redox flow batteries. It has been observed that the half-life times of those molecules are nicely correlated with redox potentials. Higher redox potentials lead to long life time or calendar life. The relationship of the molecular stability and the corresponding cycling performance was also investigated. Read More
Latest Updates
-
You’re Invited - JCESR and Beyond: Translating the Basic Science of Batteries
Please join us at Argonne National Laboratory on Tuesday, April 4, 2023 for JCESR and Beyond: Translating the Basic Science of Batteries. Registration is now open. This in-person event will celebrate 10 years of research from the Joint Center… Read More
-
A Message from JCESR: In Memory of George Crabtree
It is with heavy hearts that we say goodbye to George Crabtree, a Senior Scientist and Distinguished Fellow at Argonne National Laboratory, and Director of the Joint Center for Energy Storage Research (JCESR), who passed away unexpectedly on January 23. Dr. Read More
-
Cyanopyridines As Extremely Low-Reduction-Potential Anolytes for Nonaqueous Redox Flow Batteries
Discovery of a cyanophenylpyridine derivative with a very low reduction potential and good stability during cycling. Read More
-
Characterizing Redoxmer – Electrode Kinetics Using a SECM-Based Spot Analysis Method
Identified asymmetries in electron transfer (ET) kinetics between the reduction and oxidation of ferrocene-based redoxmers by measuring the ET rate constants (kf/kb) as a function of electrode potential. Read More
-
Benzotriazoles as Low Potential Anolytes for Non-Aqueous Redox Flow Batteries
We developed an easy-to-synthesize benzotriazole-based anolyte with a high energy redox potential (-2.3 V vs Fc/Fc+) and high solubility that demonstrates stable electrochemical cycling performance. Read More

