Chemical Transformation
-
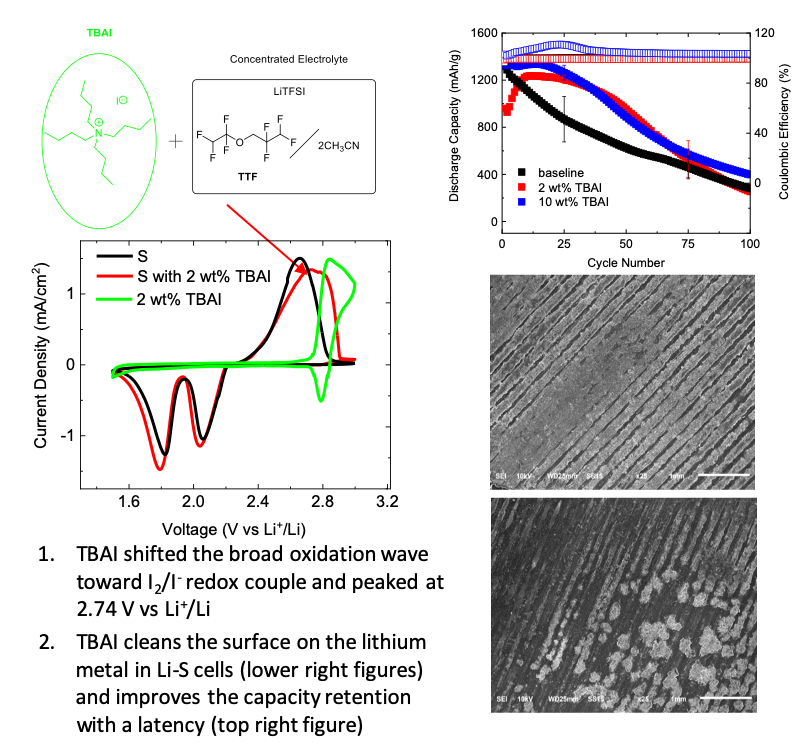
Lipophilic Additives for Highly Concentrated Electrolytes in Lithium-Sulfur Batteries
Developed a lipophilic additive, tetrabutylammonium iodide (TBAI), for highly concentrated electrolytes and tested its electrochemical performance in lithium-sulfur batteries. Read More
-
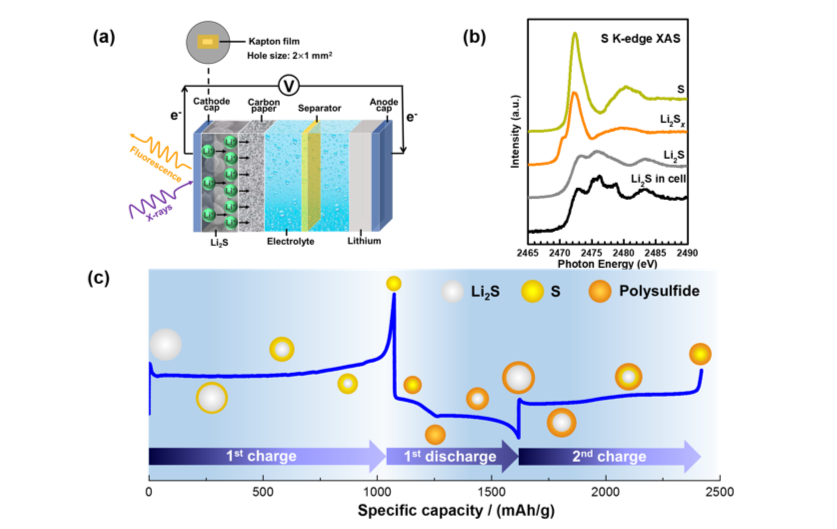
Understanding the Reaction Mechanism of Lithium-Sulfur Batteries by In situ/Operando XAS
We presented the recent progress in the application of in situ/operando XAS in the understanding of the redox mechanism of lithium sulfur batteries, which is of great significance for the development of high-performance lithium sulfur batteries. Read More
-
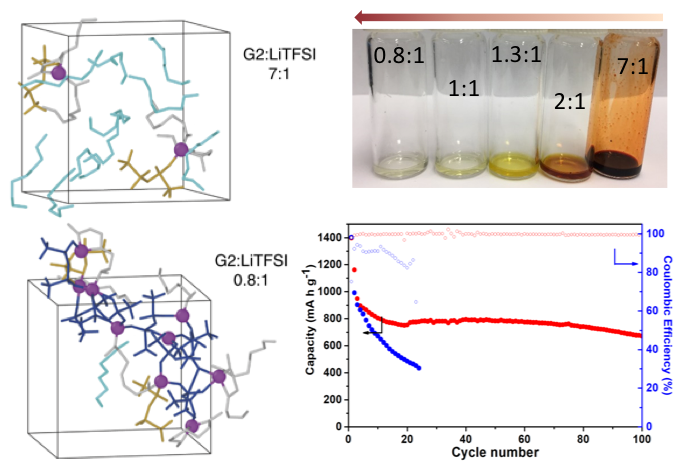
Tuning Lithium-Sulfur Battery Electrolytes for Improved Performance
The electrolyte of the lithium-sulfur (Li-S) battery was tuned to both overcome the need for a high electrolyte/sulfur ratio and inhibit the lithium dendrite growth on the anode at the same time. Read More
-
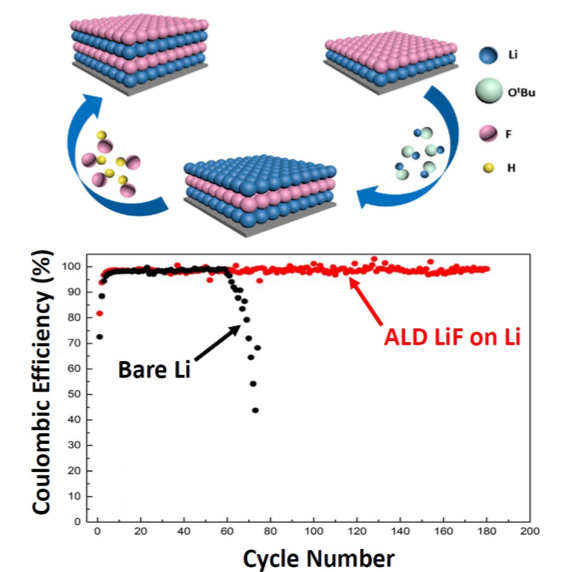
Novel ALD Chemistry Enabled Low-Temperature Synthesis of Lithium Fluoride Coatings for Durable Lithium Anodes
The team developed an atomic layer deposition (ALD) method to deposit lithium fluoride (LiF) films on lithium metal anodes. These LiF films enable a stable Coulombic efficiency as high as 99.5% for over 170 cycles, about 4 times longer than that of bare lithium anodes. Read More
-
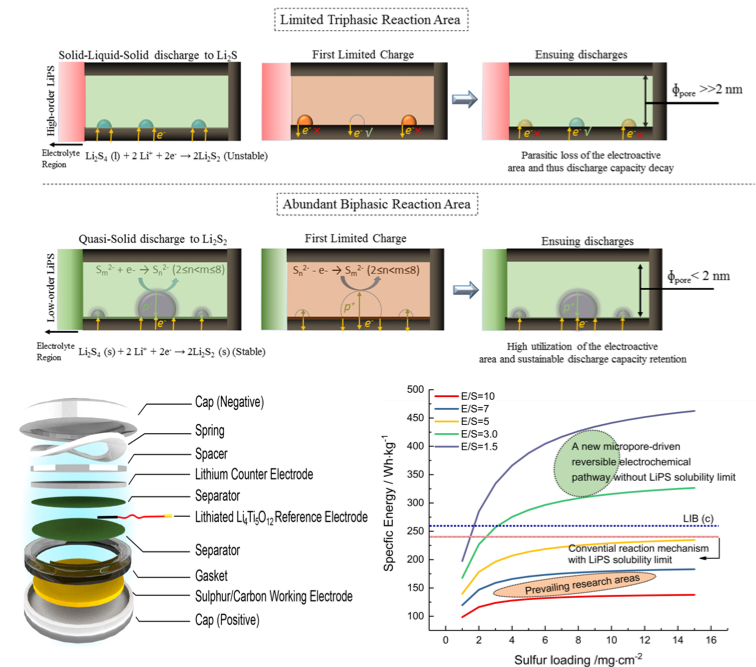
Tailored Reaction Route by Micropore Confinement for Li-S Batteries under Lean Electrolyte Conditions
Li2S2 is experimentally verified by ex-situ S K‐edge X‐ray absorption spectroscopy to be stable in the micropores during discharging in Li-S cells. A new reversible electrochemical pathway is thus proposed, based on the inherent self‐healing capacity for Li2S2, under lean electrolyte conditions. Read More
-
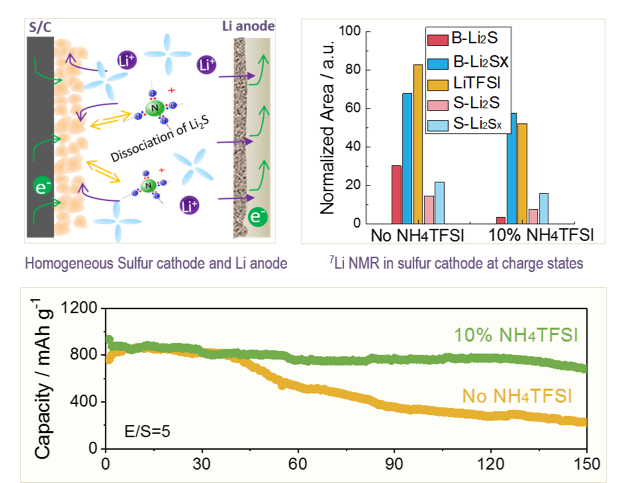
Addressing Passivation in Lithium-Sulfur Battery Under Lean Electrolyte Condition
Identification and understanding of cycle life limiting factors of Li-S batteries under lean electrolyte conditions; Identification of a NH4TFSI additive to effectively mitigate the uncontrollable passivation issue arising from accumulation of insulating Li2S. Read More
-
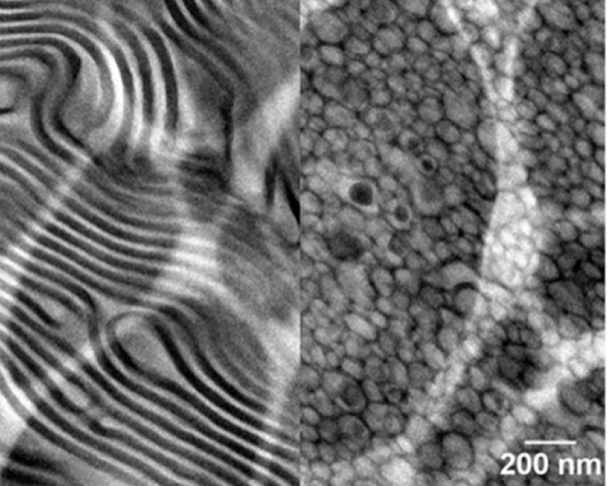
Nanostructured Electrolytes for Stabilizing Lithium Metal Anodes
The addition of salt to block copolymers results in the spontaneous and surprising formation of well-ordered lamellae in a sample that originally had a disordered morphology. Read More
-
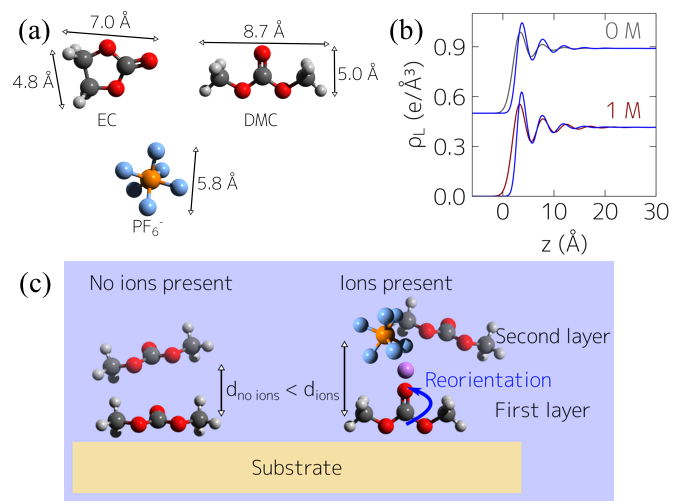
The Nanoscale Structure of the Electrolyte-metal Oxide Interface
Observation of molecular layering and insight into Li-ion salt concentration dependence of molecular orientation at metal-oxide electrolyte interfaces relevant to Li-ion batteries. Read More
-
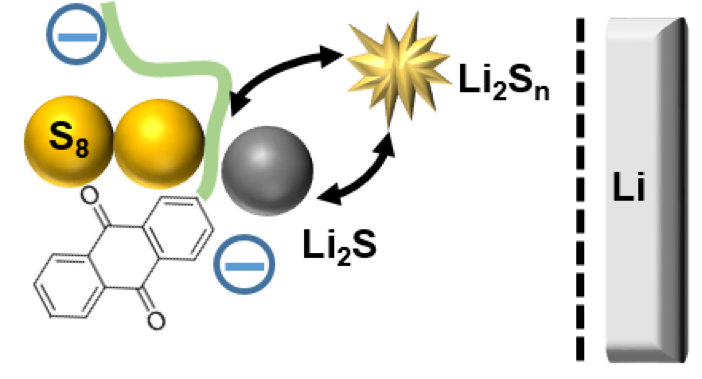
Improved Performance through Tight Coupling of Redox Cycles of Sulfur and 2,6-Polyanthraquinone in Lithium-Sulfur Batteries
We showed that the incorporation of an all-organic redox-active polymer 2,6-polyanthraquinone (PAQ) into the cathode improves capacity retention in galvanostatic cycling and inhibits Li corrosion and S deposition. Redox reactions of this polymer are shown to be strongly coupled to S redox cycle. Read More
-
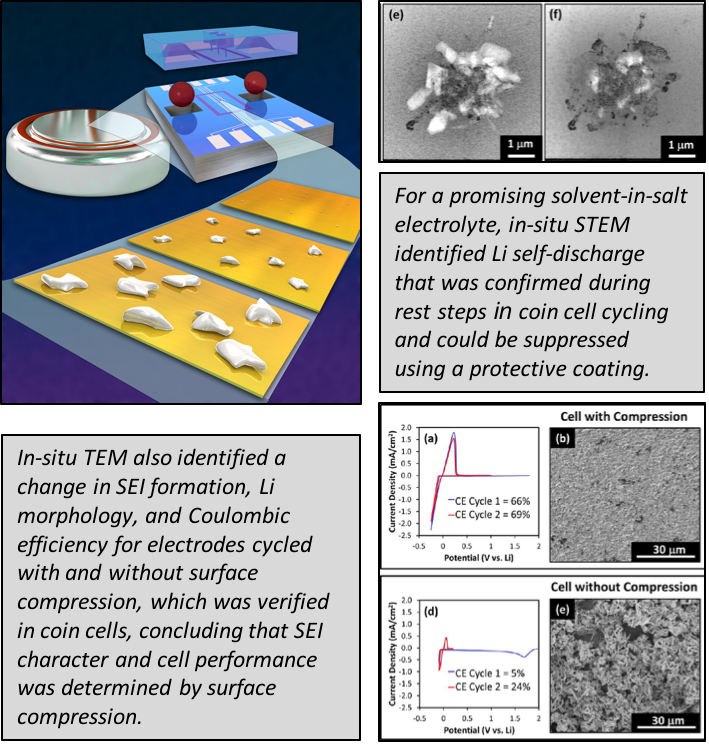
Lithium Self-Discharge and its Prevention: Direct Visualization through In-Situ Electrochemical STEM
We show that Li anode morphology and solid electrolyte interphase structure is dependent on surface compression, which affects the amount of self-discharge for an exciting solvent-in-salt electrolyte. Additionally, we show that coatings can suppress self-discharge. Read More
Latest Updates
-
You’re Invited - JCESR and Beyond: Translating the Basic Science of Batteries
Please join us at Argonne National Laboratory on Tuesday, April 4, 2023 for JCESR and Beyond: Translating the Basic Science of Batteries. Registration is now open. This in-person event will celebrate 10 years of research from the Joint Center… Read More
-
A Message from JCESR: In Memory of George Crabtree
It is with heavy hearts that we say goodbye to George Crabtree, a Senior Scientist and Distinguished Fellow at Argonne National Laboratory, and Director of the Joint Center for Energy Storage Research (JCESR), who passed away unexpectedly on January 23. Dr. Read More
-
Cyanopyridines As Extremely Low-Reduction-Potential Anolytes for Nonaqueous Redox Flow Batteries
Discovery of a cyanophenylpyridine derivative with a very low reduction potential and good stability during cycling. Read More
-
Characterizing Redoxmer – Electrode Kinetics Using a SECM-Based Spot Analysis Method
Identified asymmetries in electron transfer (ET) kinetics between the reduction and oxidation of ferrocene-based redoxmers by measuring the ET rate constants (kf/kb) as a function of electrode potential. Read More
-
Benzotriazoles as Low Potential Anolytes for Non-Aqueous Redox Flow Batteries
We developed an easy-to-synthesize benzotriazole-based anolyte with a high energy redox potential (-2.3 V vs Fc/Fc+) and high solubility that demonstrates stable electrochemical cycling performance. Read More

