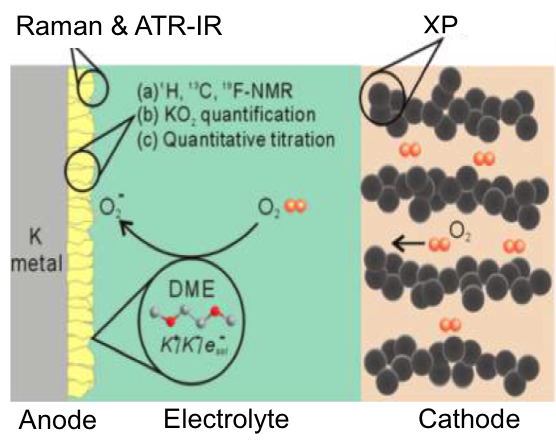
Scientific Achievement
- First comprehensive study of side reactions that dictate the fundamental electrochemistry of K-O2 battery has been carried out
- Systematic study of electrolyte reductive decomposition on the anode side with the presence of O2 cross-over carried out based on Density Functional Theory (DFT)
- Incorporation of a polymeric K+ selective membrane (i.e. Nafion-K+) as a battery separator can significantly improve the battery cycle life
Significance and Impact
- Electrolyte decomposition at the cathode in K-O2 battery is not as severe as compared to Li-O2 battery
- Introduction of a robust polymeric cation selective membrane is crucial to achieve long term operation of K-O2 battery
Research Details
- Experimental analysis and techniques that used: ATR-IR, EPR, SEM, Raman, NMR (1H, 13C, 19F), UV-vis, volumetric titration, XPS, galvanostatic
- Theoretical study is carried out using density functional theory with the B3LYP hybrid functional
This work is a collaboration between Argonne National Laboratory (JCESR managing partner), Ohio State University and HONDA Research Institute USA X. Ren, K.C. Lau, M. Yu, X. Bi, E. Kreidler, L.A. Curtiss, and Y. Wu, ACS Appl. Mater. Interfaces, 2014, 6, 19299-19307.
DOI: 10.1021/am505351s

