Scientific Achievement
Discovered why the salt LiTFSI — when added to the electrolyte of a Li-S battery — allows the battery to hold a charge much longer than other salts
Significance and Impact
Identified one reason behind Li-S batteries failing to hold a charge for long and quickly losing their charge/discharge capacity; offers needed design principles for creating long-lasting, high-capacity batteries
Research Details
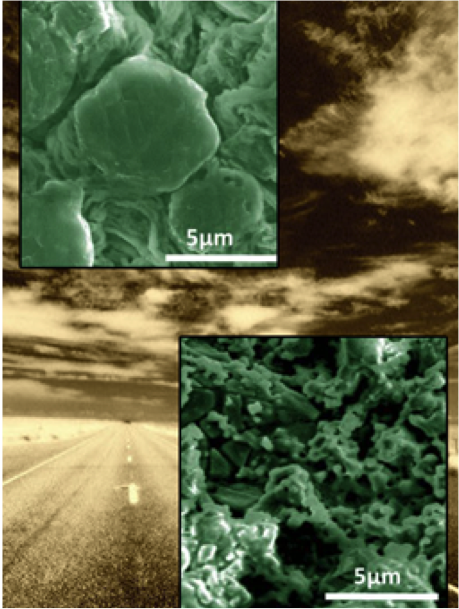
- Conducted macroscopic compositional analysis and simulations to determine the effect of LiTFSI and LiFSI on the electrolyte
- Discovered that with the LiTFSI, lithium sulfide (LiSx) forms on the electrode’s surface; when LiFSI is used, lithium sulfate (LiSOx) formed
- Calculated the bond strength and found that LiSx easily releases lithium, but LiSOx does not
Work performed at Pacific Northwest National Laboratory (JCESR partner) by Ruiguo Cao, Junzheng Chen, Kee Sung Han, Wu Xu, Donghai Mei, Priyanka Bhattacharya, Mark H. Engelhard, Karl T. Mueller, Jun Liu, and Ji-Guang Zhang, Advanced Functional Materials, 2016.

