Scientific Achievement
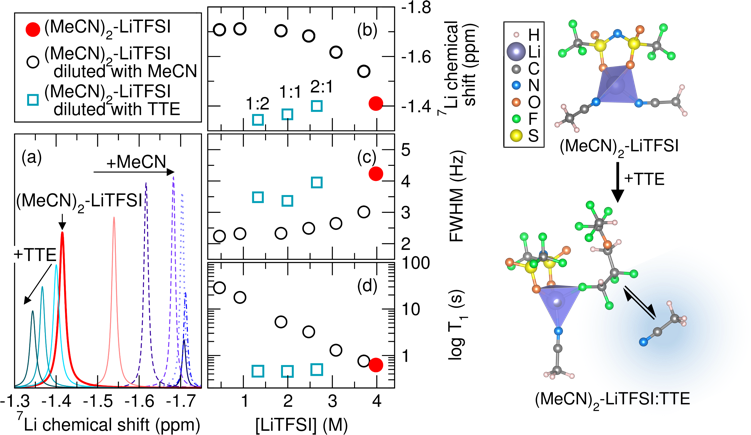
The hydrofluoroether, TTE, competes with MeCN coordination to Li+ in the solvate electrolyte resulting in a higher free MeCN content as TTE is added. The content of free MeCN affects S8 reduction kinetics likely through facilitation of polysulfide formation and enhanced local solvation effects.
Significance and Impact
The electrolyte can strongly affect the S8 reduction kinetics and therefore the battery cycling behavior. These effects can be tuned by both the structure and content of the cosolvent (hydrofluorether).
Research Details
- The reduction kinetics of S8 in the solvate electrolyte with and without TTE were evaluated by in situ Raman spectroscopy.
- The change in the local structure of Li+ in the solvate electrolyte as a result of TTE addition was probed by Raman spectroscopy coupled with 7Li NMR.
- Ab initio molecular dynamics confirm the change in the Li+ coordination as a result of TTE addition.

