Scientific Achievement
Insights are obtained into the mechanisms of adding Cs+ to protect the Li-metal electrode during battery cycling.
Significance and Impact
The addition of Cs+ can significantly enhance both the formation of well aligned Li nanorods and the reversibility of the Li electrode. In situ 133Cs NMR directly confirms that Cs+ migrates to the Li electrode to form a positively charged electrostatic shield during the charging process.
Research Details
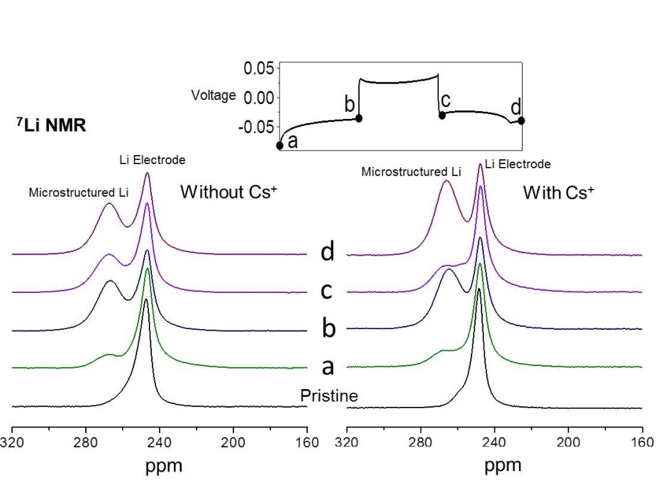
- Special symmetric Li planar cells similar to commercial coin cells (except that the stainless container was replaced by a plastic holder) were fabricated for in situ 7Li and 133Cs NMR investigations.
- The effect of the Cs additive on Li deposition onto Li-metal electrodes was investigated using quantitative in situ 7Li and 133Cs NMR.
- In situ 7Li NMR showed that a decreased amount of dendrites were formed on Li electrodes during cycling for the battery with Cs additives.
- In situ133Cs NMR indicated that Cs+ migrated to the Li electrode during the charging process.

