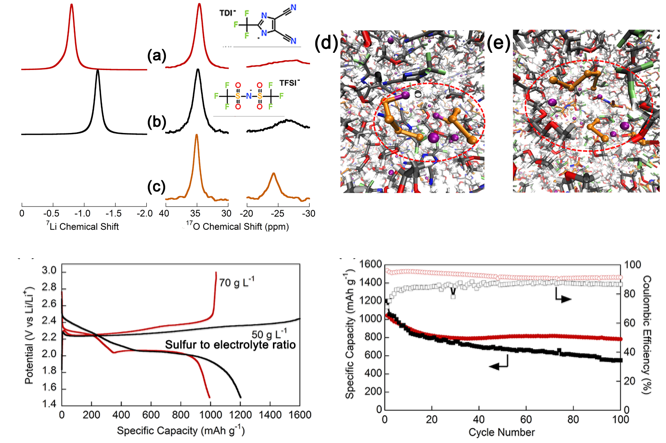
Scientific Achievement
Lithium 2-trifluoromethyl-4,5-dicyanoimidazole (LiTDI) as a supporting salt in electrolytes suppresses the maximum solubility of Li2S8 by forming a Li4S8 dimer rather than the Li2S3 and Li2S5 observed in a LiTFSI electrolyte, which enables a cell with a high sulfur loading (3 mg-S cm-2) to deliver a 1.67 mAh cm-2 areal capacity after 300 stable cycles at a high current density (2.4 mA cm-2).
Significance and Impact
LiTDI would be a suitable standard electrolyte for the lithium sulfur community since it has an improved cycling stability with both cathode and anode sides of Li-S cell.
Research Details
- NMR and DFT calculation indicated a higher Li+ solvation mainly with the DME in a standard DOL/DME mixture, which is different in the dilute solution from that of the concentrated pure DME solution.
- AIMD simualtion reveals that the TDI anion coordination affects the polysulfide disproportion to form Li4S8 dimer while reducing the solubility.
- With the incorporation of 0.1% Li2S8 and 0.2 M LiNO3 additives, excellent Li-S cell performance with cathodes having a high sulfur loading (3 mg-S cm-2) was obtained using the LiTDI-based electrolyte.

