Research Highlights
-
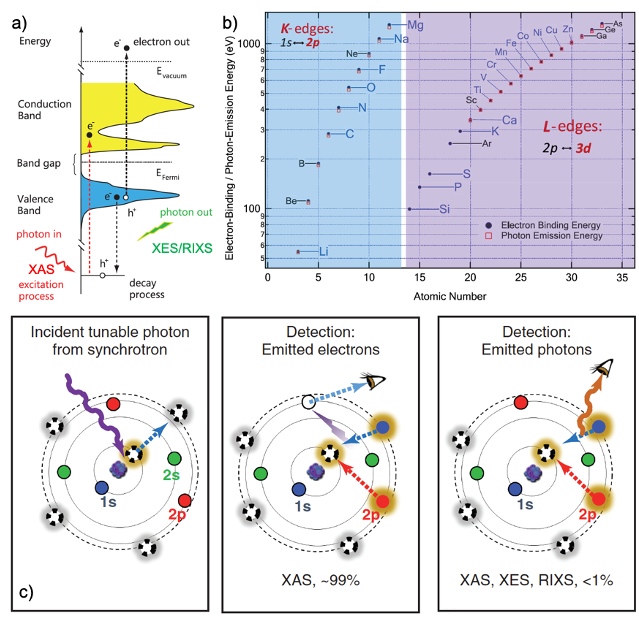
In situ/operando (soft) X-ray spectroscopy study of beyond lithium-ion batteries
The application of in situ/operando (soft) X-ray spectroscopy in beyond lithium-ion batteries is reviewed to demonstrate how such spectroscopic characterizations could facilitate the interpretation of interfacial phenomena under in-situ/operando conditions and subsequent development of the beyond lithium-ion batteries. Read More
-
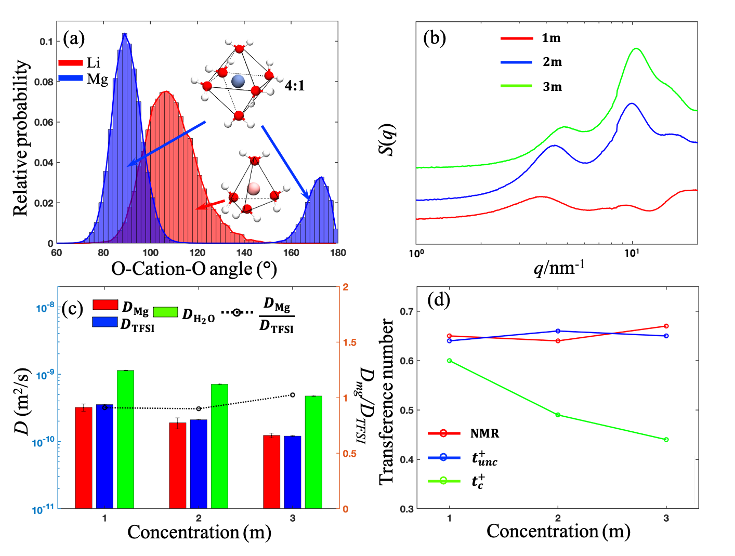
Solvation Structure and Dynamics of Mg(TFSI)2 Aqueous Electrolyte
Using MD simulations, SAXS, and PFG-NMR, the solvation structure and ion dynamics of Mg(TFSI)2 aqueous electrolytes were investigated. We found the rigid solvation structure around Mg ion increases the effective ion size, which further leads to comparable diffusivity of Mg and TFSI ion, in contrast to the LiTFSI aqueous systems where the Li ion dynamic is faster than the anion. The correlated transference numbers for Mg ions are much lower than the uncorrelated ones even at a low concentration, suggesting the enhanced correlations between ions in the multivalent electrolytes. Read More
-
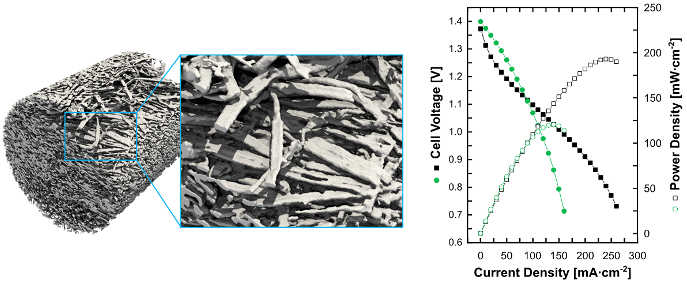
Fabrication of high surface area ribbon electrodes for use in redox flow batteries via coaxial electrospinning
This work describes a simple and reliable method to electrospinning fibers with a flat ribbon-like morphology with increased surface area to volume ratio. The new materials outperformed commercial materials at low-to-moderate current density in an operating flow cell. Read More
-
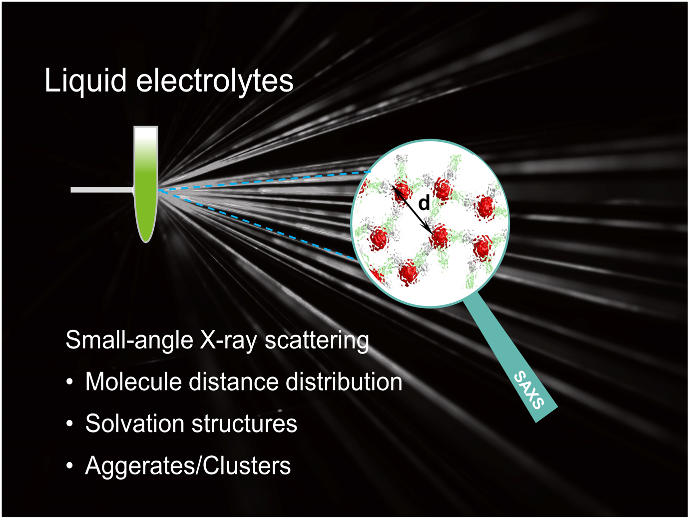
Insights into the Nanostructure, Solvation, and Dynamics of Liquid Electrolytes through Small‐Angle X‐Ray Scattering
We discussed the theory of the SAXS technique and SAXS data collection, processing, and analysis of liquid electrolytes. Also, the recent developments in understanding liquid electrolytes are summarized, including: the characterization of solvation structures, the liquid structures in a water-in-salt electrolyte, concentrated electrolyte, localized concentrated electrolyte, and ionic liquids. Read More
-
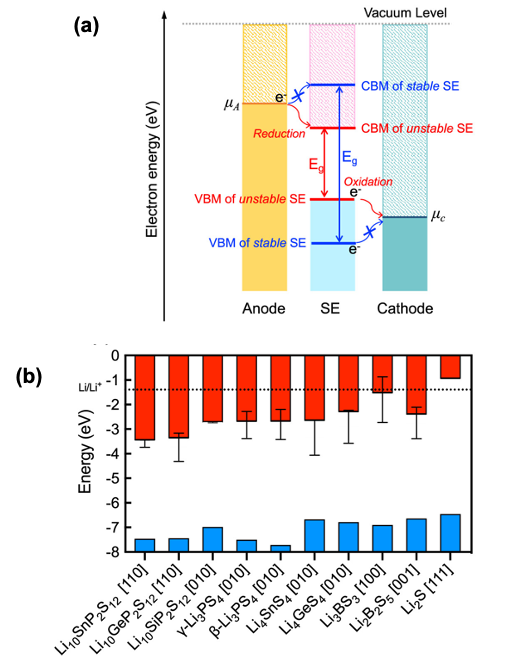
Predicting Charge Transfer Stability Between Sulfide Solid Electrolytes and Li Metal Anodes
The likelihood for charge injection from a Li metal anode to 10 sulfide-based SEs was determined by computing the positions of the SE’s band edges with respect to the electrochemical potential of the electrode. Trends in charge transfer stability were compared to those for chemical stability with a Li anode and were found to be similar. The combined characterization of chemical and charge transfer phenomena allows for a comprehensive assessment of interfacial stability. Read More
-
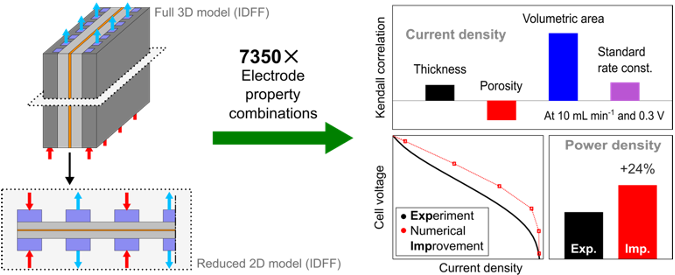
Data-driven electrode parameter identification for vanadium redox flow batteries through experimental and numerical methods
This study provides extensive validation for 3D-to-2D model reduction for redox flow batteries (RFBs). This computationally light, 2D model is used to generate a data set of >6,000 unique RFB simulations for statistical quantification. Read More
-
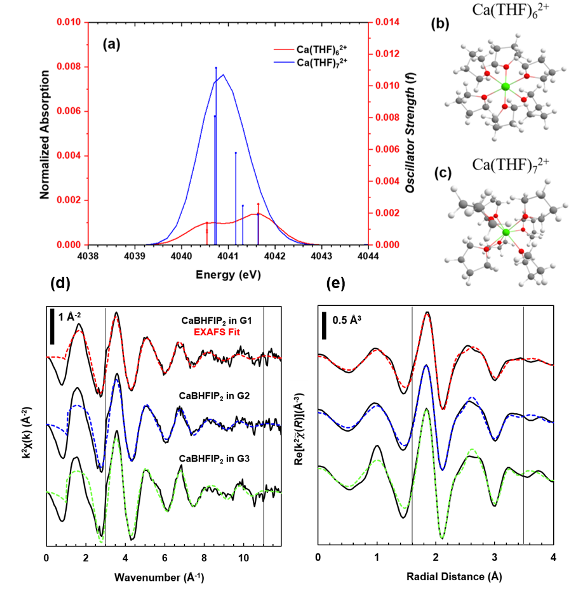
Quantifying Ethereal Solvation Effects on Ca2+ Coordination in Well-Dissociated Electrolytes
Through the combination of X-ray absorption fine structure (XAFS) and time-dependent density functional theory (TDDFT), descriptive measures of the local geometry, coordination, and electronic structure of Ca–ethereal complexes provide distinct structural trends depending on the extent of the Ca2+–solvent interaction. Read More
-
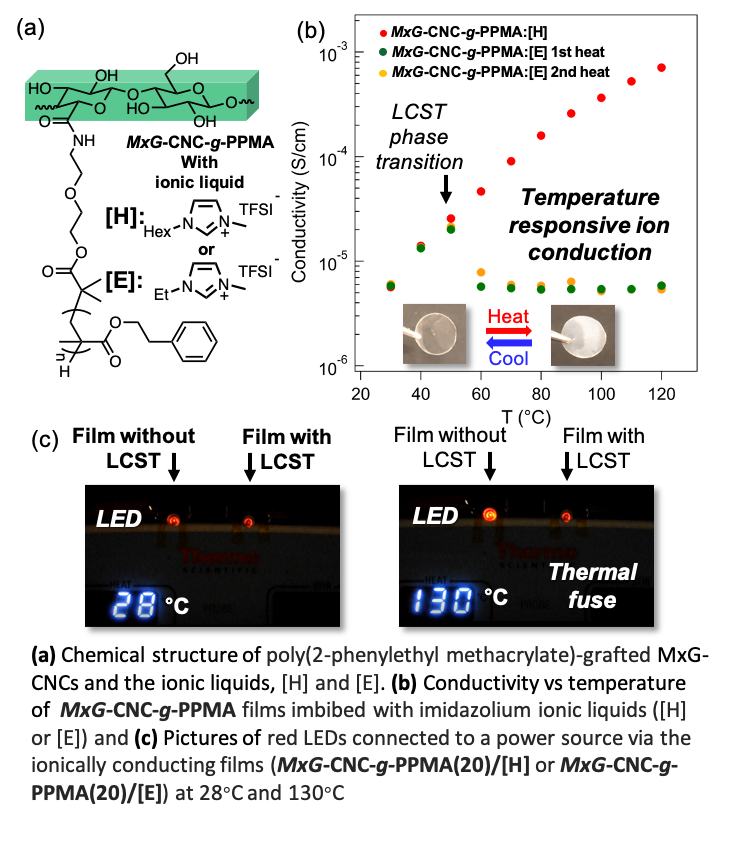
Ion-conducting Thermo-responsive Films Based on Polymer Grafted Cellulose Nanocrystals
Mechanically robust, thermoresponsive, ion-conducting nanocomposite films have been prepared from ionic liquid imbibed poly(2-phenylethyl methacrylate)-grafted cellulose nanocrystals. On account of the lower critical solution temperature (LCST) of the grafted polymer in the ionic liquid, these materials exhibit a conductivity decrease around 60 °C Read More
-
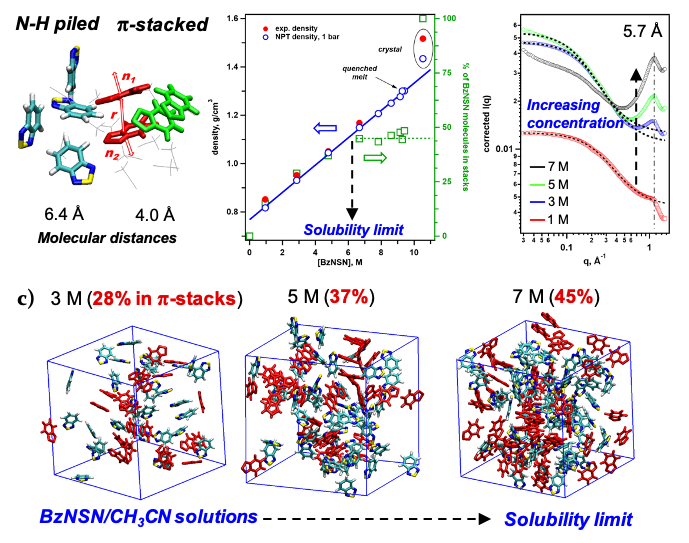
Competition of Stacking and Piling Improves Molecular Solubility in Electrolyte
Organic redox-active molecules (redoxmers) are charge carriers in redox flow cells. Since the energy density of a battery fluid is proportional to concentration of active molecules, high molecular solubility is desirable. However, as the redoxmer solutions become crowded, solute-solute interactions become stronger, opposing high solubility. Here we demonstrate how a small redoxmer molecule, 2,1,3-benzothiadiazole (BzNSN), can achieve exceptionally high solubility through a competition of two packing motifs – N-H bond piling and π-stacking – that introduces disorder that frustrates crystallization in concentrated solutions. Read More
-
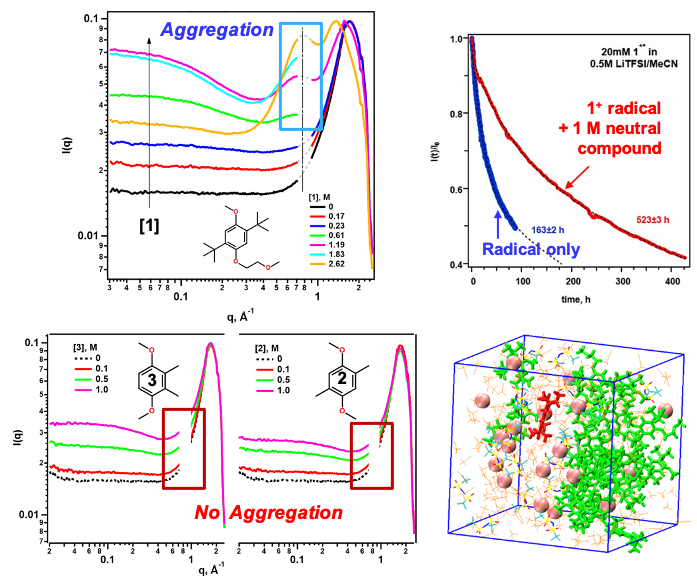
Greater redoxmer stability through nanoconfinement
Structural diversity of organic redox-active molecules (redoxmers) permits tuning not only solute-electrolyte interactions but also solute-solute interactions in concentrated electrolyte solutions. The 1,4-dimethoxybenzene (DMB) family is an example of such flexibility. We show that DMB molecule 1 forms extended aggregates in concentrated solutions whereas simpler DMB molecules 2 and 3 do not. The resulting networks expel solvent and aggregated ions to small and increasingly isolated nanodomains. Charged redoxmer molecules are also expelled to such domains and their stability increases dramatically due to nanoconfinement (“microreactor”) effects. Read More
Latest Updates
-
You’re Invited - JCESR and Beyond: Translating the Basic Science of Batteries
Please join us at Argonne National Laboratory on Tuesday, April 4, 2023 for JCESR and Beyond: Translating the Basic Science of Batteries. Registration is now open. This in-person event will celebrate 10 years of research from the Joint Center… Read More
-
A Message from JCESR: In Memory of George Crabtree
It is with heavy hearts that we say goodbye to George Crabtree, a Senior Scientist and Distinguished Fellow at Argonne National Laboratory, and Director of the Joint Center for Energy Storage Research (JCESR), who passed away unexpectedly on January 23. Dr. Read More
-
Cyanopyridines As Extremely Low-Reduction-Potential Anolytes for Nonaqueous Redox Flow Batteries
Discovery of a cyanophenylpyridine derivative with a very low reduction potential and good stability during cycling. Read More
-
Characterizing Redoxmer – Electrode Kinetics Using a SECM-Based Spot Analysis Method
Identified asymmetries in electron transfer (ET) kinetics between the reduction and oxidation of ferrocene-based redoxmers by measuring the ET rate constants (kf/kb) as a function of electrode potential. Read More
-
Benzotriazoles as Low Potential Anolytes for Non-Aqueous Redox Flow Batteries
We developed an easy-to-synthesize benzotriazole-based anolyte with a high energy redox potential (-2.3 V vs Fc/Fc+) and high solubility that demonstrates stable electrochemical cycling performance. Read More

