Scientific Achievement
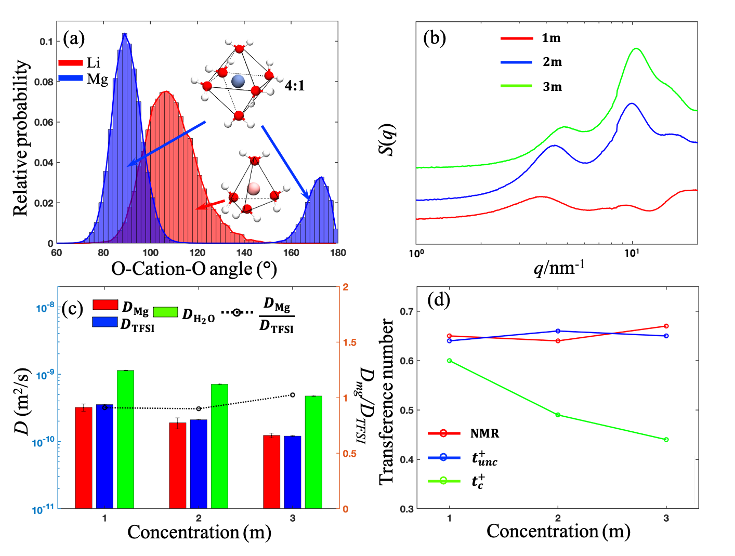
Using MD simulations, SAXS, and PFG-NMR, the solvation structure and ion dynamics of Mg(TFSI)2 aqueous electrolytes were investigated. We found the rigid solvation structure around Mg ion increases the effective ion size, which further leads to comparable diffusivity of Mg and TFSI ion, in contrast to the LiTFSI aqueous systems where the Li ion dynamic is faster than the anion. The correlated transference numbers for Mg ions are much lower than the uncorrelated ones even at a low concentration, suggesting the enhanced correlations between ions in the multivalent electrolytes.
Significance and Impact
This work provides a molecular-level understanding of how the solvation structure and multivalency of the ion affect the dynamics and transport properties of the multivalent electrolyte, providing insight for rational designs of electrolytes for improved ion transport properties.
Research Details
- The nanostructure of Mg(TFSI)2 aqueous electrolytes were investigated using MD simulation and SAXS technique. Compare to Li ion, the octahedral solvation shell around Mg is more rigid.
- The dynamics of of Mg(TFSI)2 aqueous electrolytes were investigated using MD simulation and PFG-NMR techniques. We found the self diffusivity of Mg and TFSI are comparable.
- The correlated transference number and diffusivity-viscosity relationship deviate from the Stokes-Einstein relation, which highlights the significant ionic correlation in the multivalent electrolytes.

