Research Highlights
-
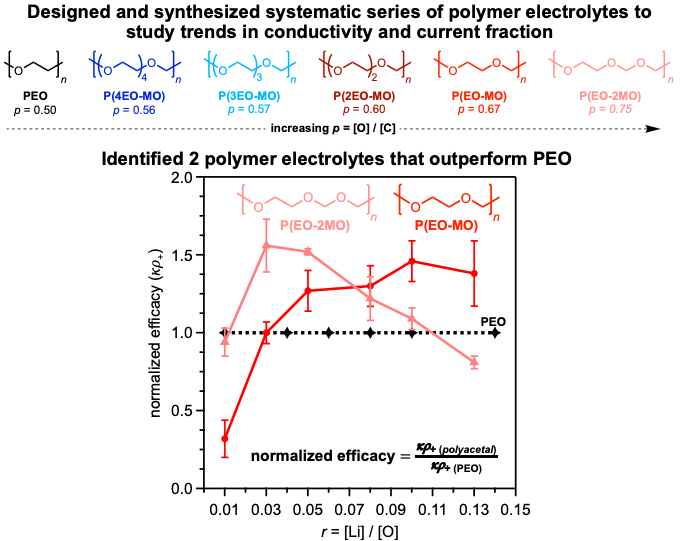
Improved Li+ transport in Polyacetal Electrolytes: Conductivity and Current Fraction in a Series of Polymers
Our team identified several polymer electrolytes that outperform Li-ion transport in PEO by nearly 150% based on conductivity and current fraction measurements. Read More
-
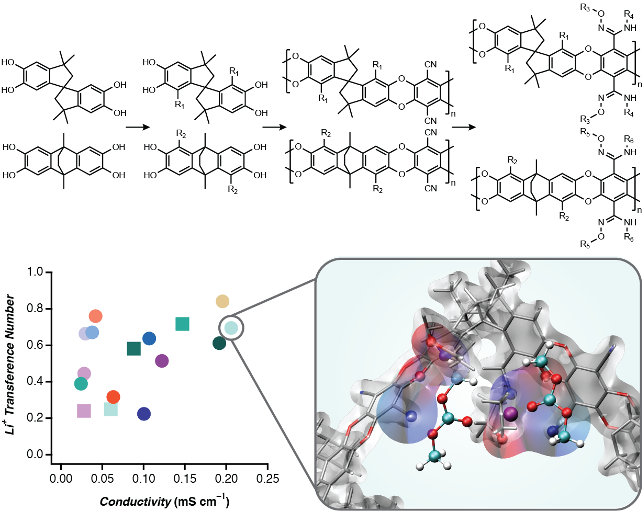
Diversity-oriented synthesis of polymer membranes with ion solvation cages
Diversification of the PIM scaffold accelerates the identification of PIMs with desirable guest-host interactions. Li-solvating morpholine groups displayed in the PIM’s free volume elements offer exceptionally high conductivity and LI+ transference number. Read More
-
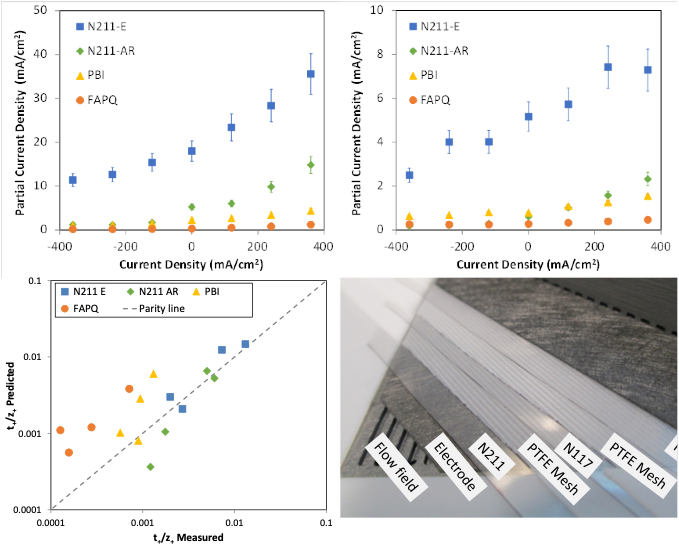
The Influence of Current Density on Transport of Vanadium Cations through Membranes with Different Charges
Evaluated the transference numbers of four vanadium cations through positively, negatively, and initially uncharged membranes. Complete data sets collected including conductivity, transference number, and sorption. Read More
-
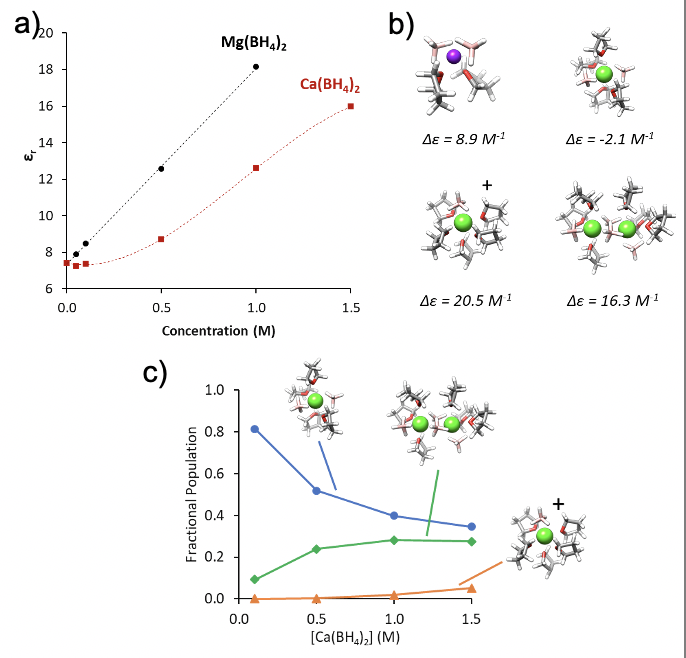
Quantifying Species Populations in Multivalent Borohydride Electrolytes
The relative concentrations of the critical species present in complex borohydride electrolytes were determined, quantifying solvation environment evolution and species formation as a function of which multivalent cation (Mg or Ca) is present. Read More
-
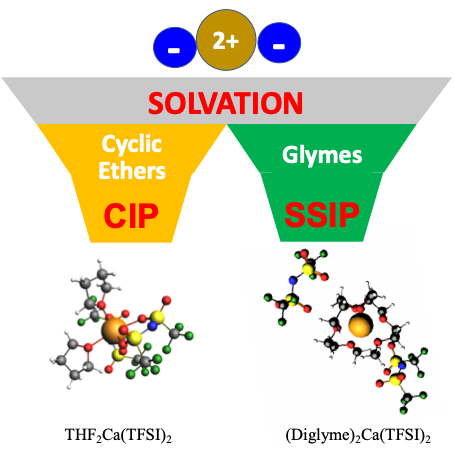
Factors Influencing Preferential Anion Interactions during Solvation of Multivalent Cations in Ether-based Solvents
Discovered and described how solvent structure and cation size influence cation−anion interactions, suggesting that they drive the formation of free ions in ethereal multivalent electrolytes. Read More
-
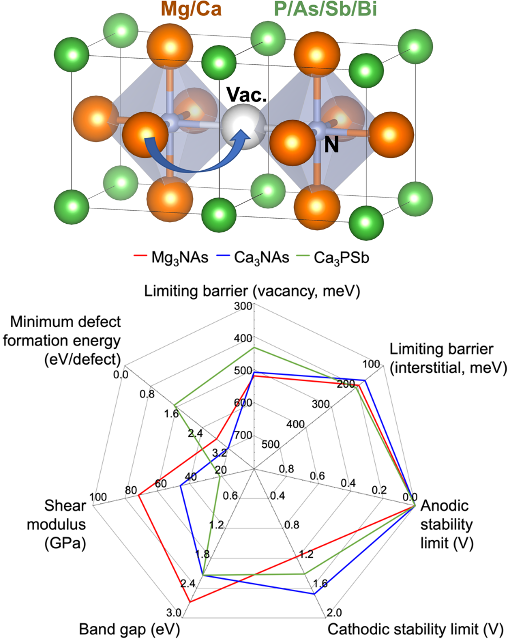
Multivalent Ion Transport in Anti-Perovskite Solid Electrolytes
A comprehensive computational approach is used to discover potential multivalent (MV)-ion conducting solid electrolytes (SEs) based on the anti-perovskite (AP) crystal structure. Read More
-
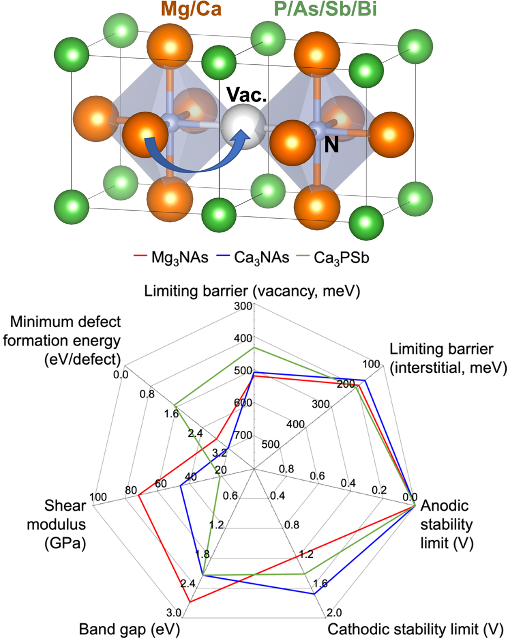
Multivalent Ion Transport in Anti-Perovskite Solid Electrolytes
A comprehensive computational approach is used to discover potential multivalent (MV)-ion conducting solid electrolytes (SEs) based on the anti-perovskite (AP) crystal structure. Read More
-
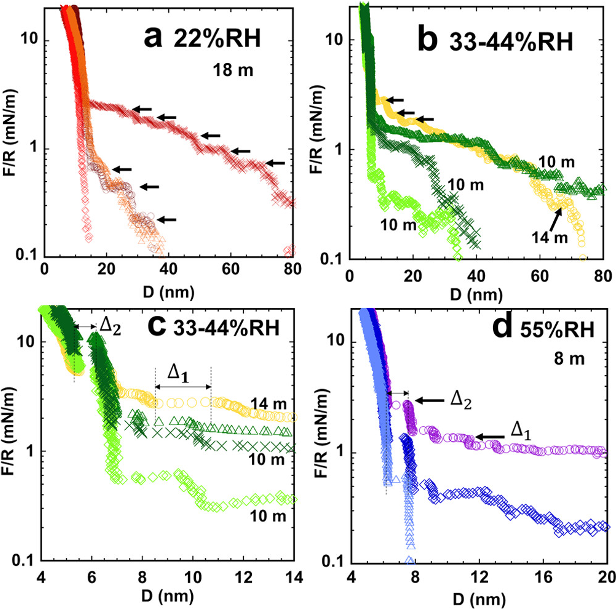
Nanoheterogeneity of LiTFSI Solutions Transitions Close to a Surface and with Concentration
The interfacial structure of the water-in-salt electrolytes (WiSE) on a mica surface is investigated. Nanostructures of 1nm to 3 nm are observed. The size of the nanostructure varies depending on the concentration of the LiTFSI/H2O system. Read More
-
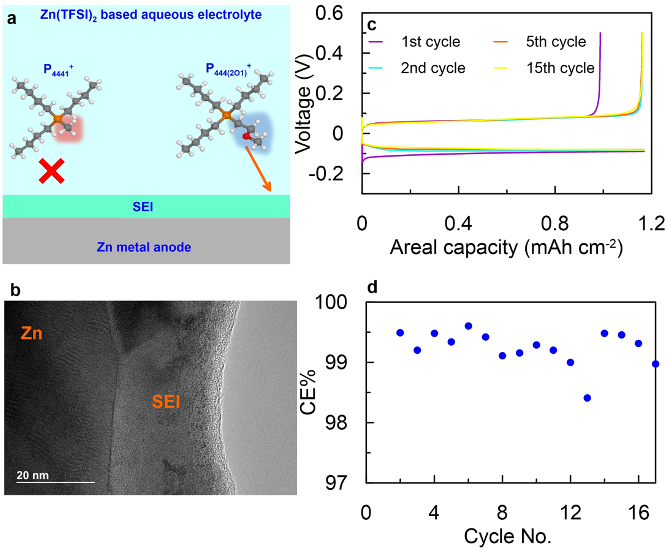
Functionalized Phosphonium Cations Enable Zn Metal Reversibility in Aqueous Electrolytes
The presence of P444(2O1)+ cation in aqueous electrolyte enhances Zn reversibility dramatically by enabling a high Coulombic efficiency (CE, > 99%) at high utilization (20% Zn per cycle) for Zn plating/stripping, suppressing hydrogen evolution, and allowing a remarkable dendrite-free cycling even under aggressive conditions (2.5 mA/cm2, 2.5 mAh/cm2). Read More
-
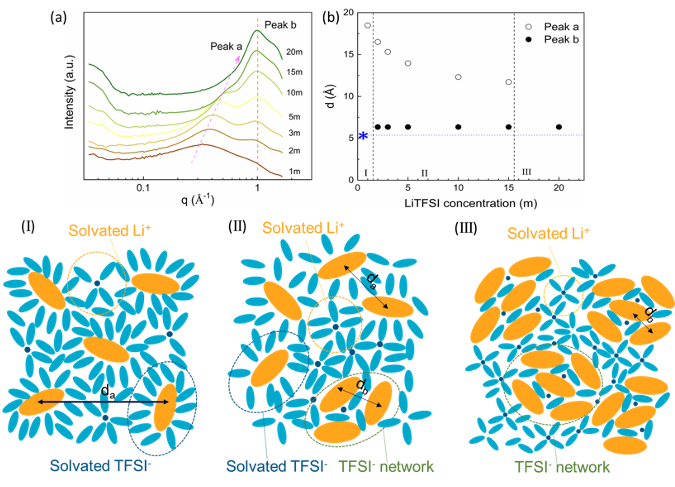
Microscopic Understanding of the Ionic Networks of “Water-in-Salt” Electrolytes
We conduct SAXS study to investigate the solvation behavior of LiTFSI aqueous solutions in a wide range of concentration from 1 to 20 m. Also, the stability of these samples under different temperatures is studied by in situ SAXS measurements. Read More
Latest Updates
-
You’re Invited - JCESR and Beyond: Translating the Basic Science of Batteries
Please join us at Argonne National Laboratory on Tuesday, April 4, 2023 for JCESR and Beyond: Translating the Basic Science of Batteries. Registration is now open. This in-person event will celebrate 10 years of research from the Joint Center… Read More
-
A Message from JCESR: In Memory of George Crabtree
It is with heavy hearts that we say goodbye to George Crabtree, a Senior Scientist and Distinguished Fellow at Argonne National Laboratory, and Director of the Joint Center for Energy Storage Research (JCESR), who passed away unexpectedly on January 23. Dr. Read More
-
Cyanopyridines As Extremely Low-Reduction-Potential Anolytes for Nonaqueous Redox Flow Batteries
Discovery of a cyanophenylpyridine derivative with a very low reduction potential and good stability during cycling. Read More
-
Characterizing Redoxmer – Electrode Kinetics Using a SECM-Based Spot Analysis Method
Identified asymmetries in electron transfer (ET) kinetics between the reduction and oxidation of ferrocene-based redoxmers by measuring the ET rate constants (kf/kb) as a function of electrode potential. Read More
-
Benzotriazoles as Low Potential Anolytes for Non-Aqueous Redox Flow Batteries
We developed an easy-to-synthesize benzotriazole-based anolyte with a high energy redox potential (-2.3 V vs Fc/Fc+) and high solubility that demonstrates stable electrochemical cycling performance. Read More

