Research Highlights
-
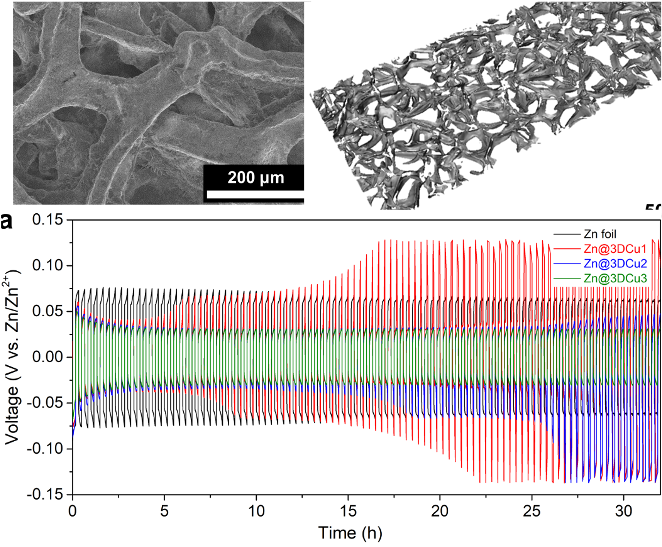
Highly Reversible Plating/Stripping of Porous Zinc Anodes for Multivalent Zinc Batteries
Highly reversible Zn plating/stripping achieved with electrodes prepared by electrodepositing Zn onto porous 3D Cu foam. Read More
-

Ion Solvation Engineering: How to Manipulate the Multiplicity of the Coordination Environment of Multivalent Ions
Advanced free-energy sampling analysis shows that the multiplicity of thermodynamically stable solvation configurations is a general feature of free divalent cations, their ion-pairs and neutral aggregates in low dielectric solvents. We reveal the macro- and microscopic factors of the solvation multiplicity and show how their interplay can be used to manipulate ion solvation environments and thus the ion solvation-desolvation dynamics. Read More
-
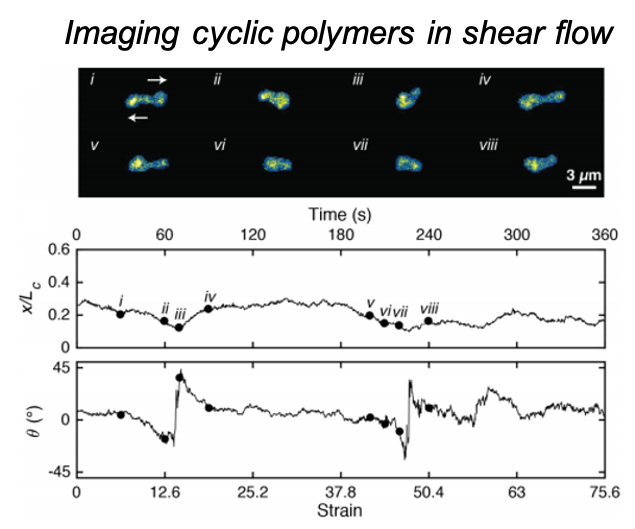
Direct observation of cyclic polymer dynamics in shear flow reveals non-equilibrium behavior for flow-based applications
Single ring polymers were directly visualized in the flow-gradient plane of shear flow to understand their dynamic behavior such as tumbling and stretching in flow, as well as the distribution of polymer conformations far-from-equilibrium. Read More
-
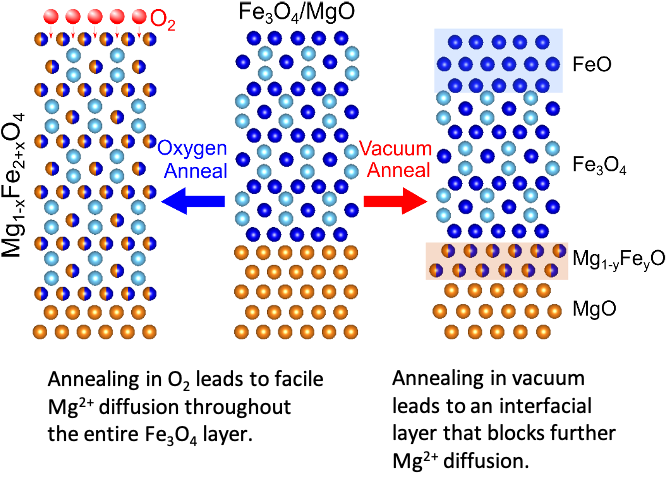
Oxygen Facilitates Mg2+ Diffusion Through Fe3O4
Controlled processing of carefully designed Fe3O4/MgO heterostructures allowed identification of the Mg2+ diffusion pathways, reaction intermediates, and the key role of the anion in these energy-relevant systems. Read More
-
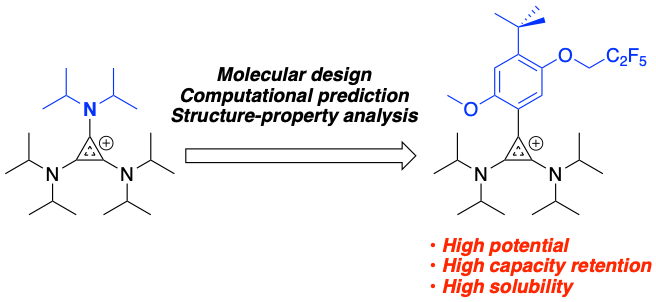
Bis(diisopropylamino)cyclopropenium-arene Cations as High Oxidation Potential and High Stability Catholytes for Nonaqueous Redox Flow Batteries
Development of a new catholyte with both extremely high potential and much improved cycling stability Read More
-
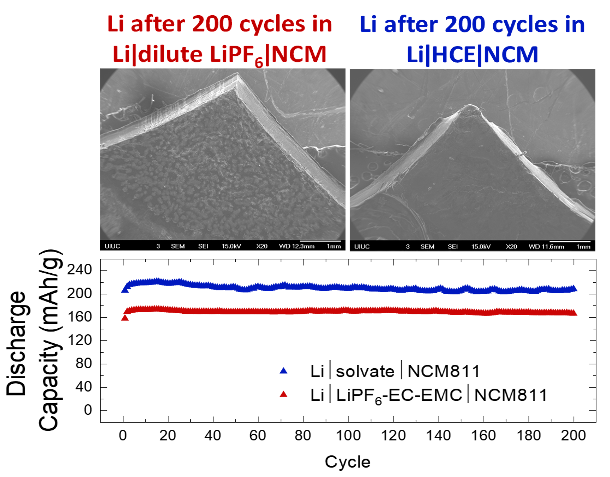
A Highly Concentrated Electrolyte for High Capacity and Coulombic Efficiency in Li-NCM811 Cells
Replacing a dilute 1 M LiPF6-based electrolyte with a hydrofluoroether (HFE)-modified highly concentrated electrolyte (HCE) stabilizes the Li metal interface and results in higher discharge capacity, Coulombic efficiency, and thermal stability. Read More
-
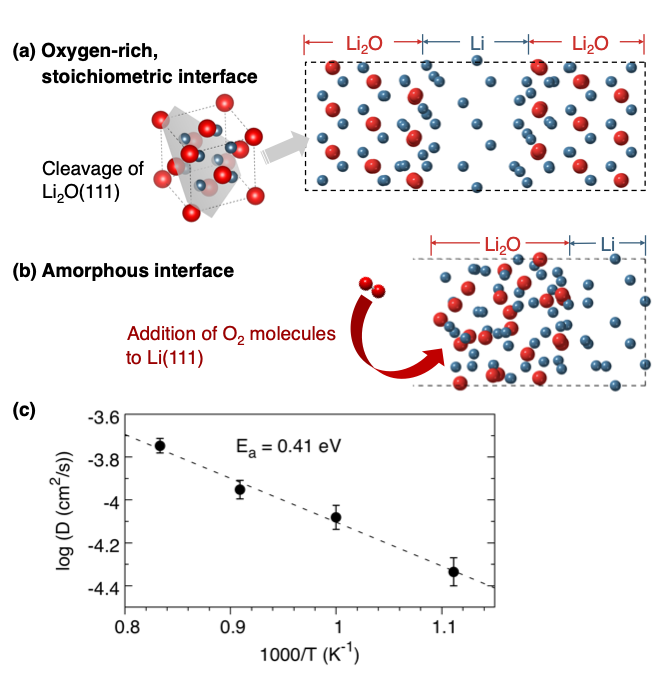
Modeling the Interface Between Lithium Metal and its Native Oxide
The transport of Li+ within the amorphous Li/Li2O interface is predicted to be roughly three orders of magnitude faster than in crystalline Li2O (as determined in the literature). Read More
-
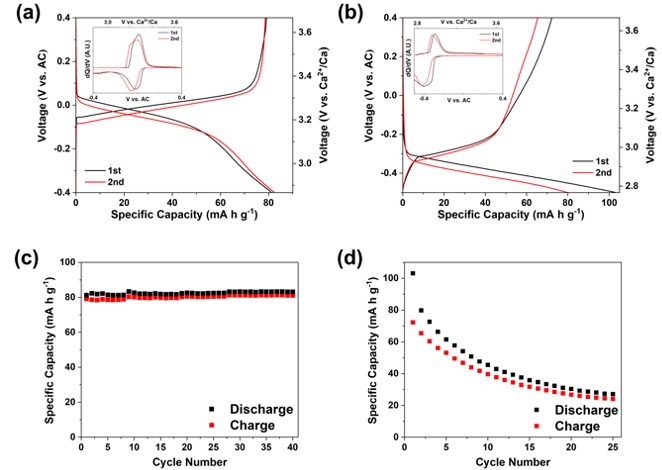
High-Voltage Phosphate Cathodes for Rechargeable Ca-Ion Batteries
Two functional polyanionic phosphate materials, NASICON-type NaV2(PO4)3 and olivine-type FePO4, are demonstrated as high voltage Ca-ion battery cathodes for the first time. Read More
-
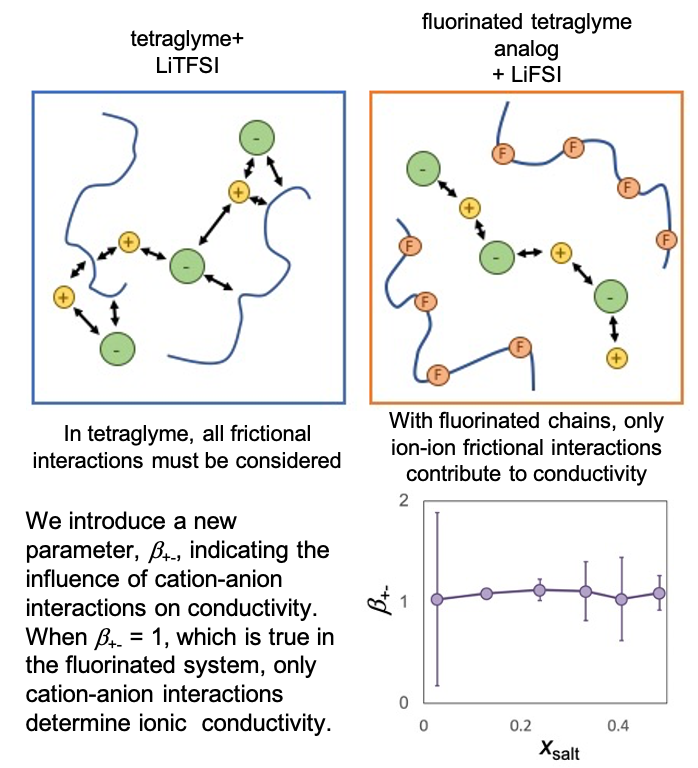
Impact of Frictional Interactions on Electrochemical Properties in Polymer Electrolytes
We introduce a new parameter, b+-, indicating the influence of cation-anion interactions on conductivity. When b+- = 1, which is true in the fluorinated system, only cation-anion interactions determine ionic conductivity. Read More
-
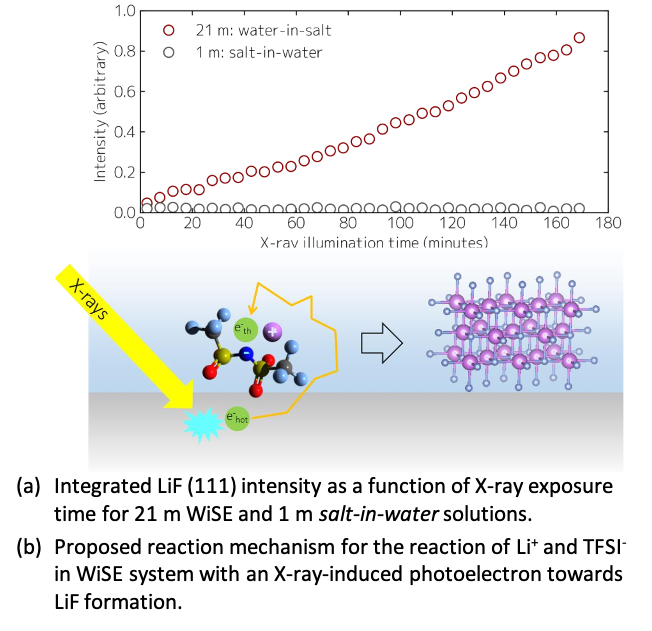
X-ray-Induced Lithium Fluoride Formation from Water-in-Salt Electrolytes on Solid Surfaces
LiTFSI/H2O electrolyte interfacial decomposition pathways in the “water-in-salt” (WiSE) and “salt in-water” regimes are investigated using synchrotron X-rays. The resultant photoelectron-induced reduction towards LiF formation was revealed to be occur among closely contact ion-pairs adjacent to the interface. This supports present models behind the WiSE concept. Read More
Latest Updates
-
You’re Invited - JCESR and Beyond: Translating the Basic Science of Batteries
Please join us at Argonne National Laboratory on Tuesday, April 4, 2023 for JCESR and Beyond: Translating the Basic Science of Batteries. Registration is now open. This in-person event will celebrate 10 years of research from the Joint Center… Read More
-
A Message from JCESR: In Memory of George Crabtree
It is with heavy hearts that we say goodbye to George Crabtree, a Senior Scientist and Distinguished Fellow at Argonne National Laboratory, and Director of the Joint Center for Energy Storage Research (JCESR), who passed away unexpectedly on January 23. Dr. Read More
-
Cyanopyridines As Extremely Low-Reduction-Potential Anolytes for Nonaqueous Redox Flow Batteries
Discovery of a cyanophenylpyridine derivative with a very low reduction potential and good stability during cycling. Read More
-
Characterizing Redoxmer – Electrode Kinetics Using a SECM-Based Spot Analysis Method
Identified asymmetries in electron transfer (ET) kinetics between the reduction and oxidation of ferrocene-based redoxmers by measuring the ET rate constants (kf/kb) as a function of electrode potential. Read More
-
Benzotriazoles as Low Potential Anolytes for Non-Aqueous Redox Flow Batteries
We developed an easy-to-synthesize benzotriazole-based anolyte with a high energy redox potential (-2.3 V vs Fc/Fc+) and high solubility that demonstrates stable electrochemical cycling performance. Read More

