Research Highlights
-
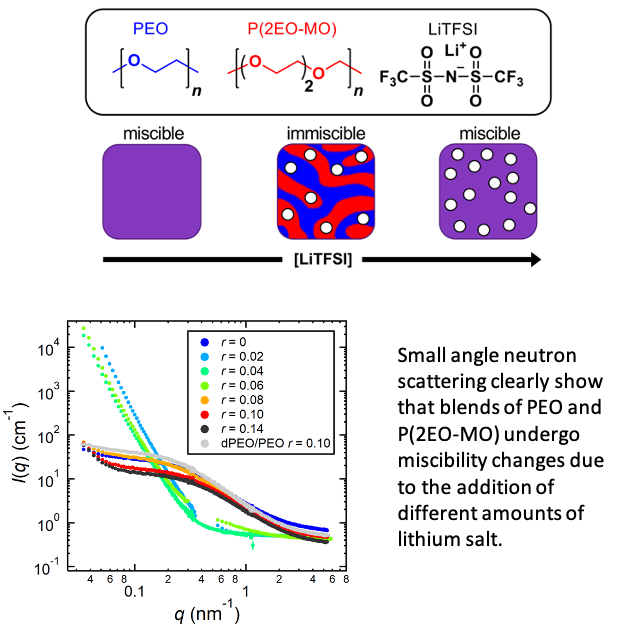
Miscible Polymer Electrolyte Blends
This work opens the door to blending different polymers to create optimal electrolytes in a manner that is similar to that used to optimize liquid electrolytes in lithium-ion batteries. Read More
-
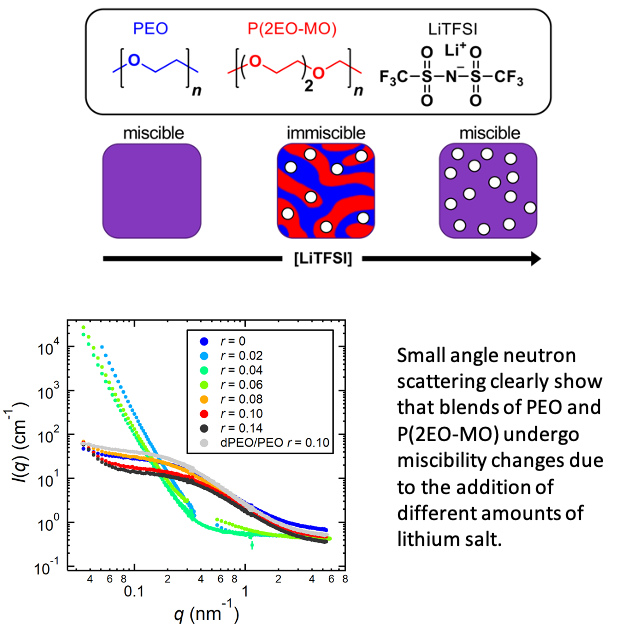
Miscible Polymer Electrolyte Blends
This work opens the door to blending different polymers to create optimal electrolytes in a manner that is similar to that used to optimize liquid electrolytes in lithium-ion batteries. Read More
-
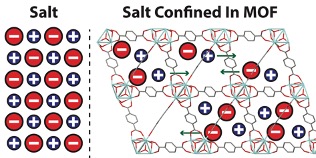
Salt nanoconfinement in zirconium-based metal organic frameworks leads to pore-size and loading-dependent ionic conductivity enhancement
The nanoscale confinement of [NEt4][TFSI] in three isostructural MOFs enhanced the conductivity of composites relative to neat [NEt4][TFSI] by up to a factor of 50. The conductivity increases with the increase in pore size and maximum conductivity was achieved with a salt loading slightly less than that required for complete filling of the MOF pores. Read More
-
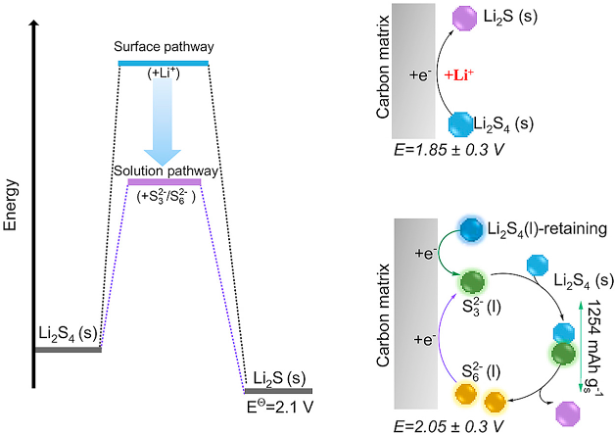
Solution-mediated reduction pathway enables Li–S cells to operate at lean electrolyte conditions
A solution-mediated reaction mechanism is proposed and confirmed for Li-S redox reactions through electrochemical and operando X-ray absorption spectroscopy characterization. Read More
-
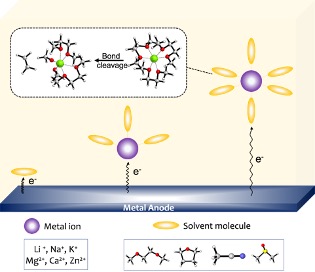
Prediction of Molecular Structures and Electron Affinities of Metal-Solvent Complexes
Simulated molecular structures and the reductive stabilities of many monovalent and divalent metal ion complexes with organic solvents. Read More
-
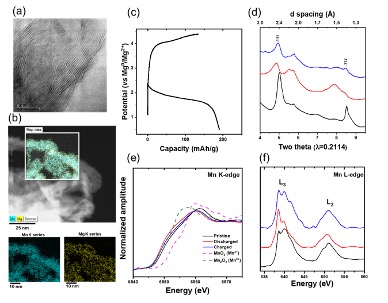
Intercalation of Mg into a Few-Layer Phyllomanganate in Non-aqueous Electrolytes at Room Temperature
Nanocrystals of a few-layer manganese oxide are shown to have considerable electrochemical activity toward Mg2+ intercalation even at room temperature, where they delivered ~190 mAh/g in full Mg metal batteries. Read More
-
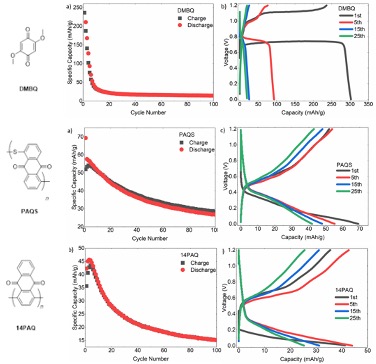
Mechanistic Insights in Quinone-Based Zinc Batteries with Nonaqueous Electrolytes
Three quinone-based organic electrodes were tested in nonaqueous Zn-batteries using different salts and solvents. Mechanistic studies on the electrode kinetics were then performed. Read More
-
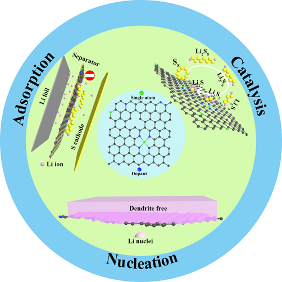
Design Principles of Single Atoms on Carbons for Lithium–Sulfur Batteries
We summarize the latest strategies for single atoms (SAs) supported on carbons for the application of Li-S batteries, including cathode, modified separator, and Li metal anode. The future directions of SAs in Li-S batteries are proposed. Read More
-
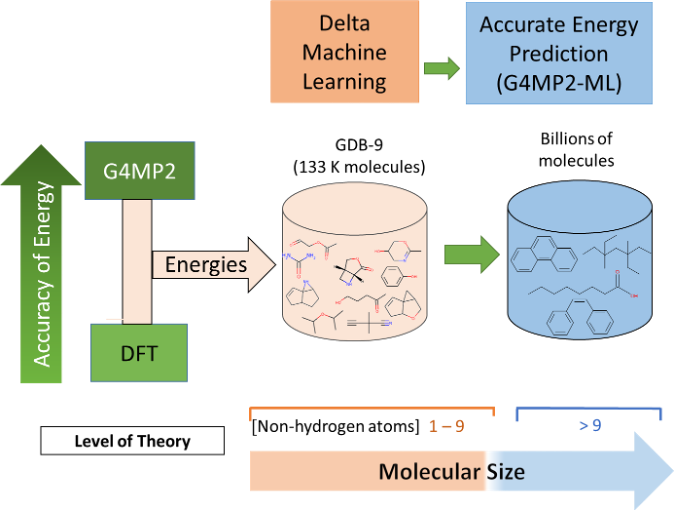
Quantum-Chemically Informed Machine Learning for Fast and Accurate Prediction of Energies of Large Molecules
This work has demonstrated that quantum chemically informed machine learning can be used to successfully predict energies of large organic molecules with sizes beyond those in the training set at a much lower cost in computer time. Read More
-
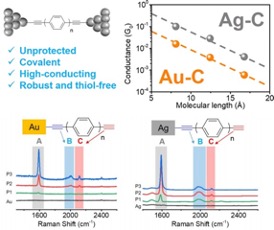
Unprotected terminal acetylenes form covalent bonding contacts with metal electrode surfaces
Unprotected acetylene groups were found to form a simple new electrode-anchor pair (silver-acetylene) for forming robust, low resistance contacts for mediating or controlling molecule-electrode interactions. Read More
Latest Updates
-
You’re Invited - JCESR and Beyond: Translating the Basic Science of Batteries
Please join us at Argonne National Laboratory on Tuesday, April 4, 2023 for JCESR and Beyond: Translating the Basic Science of Batteries. Registration is now open. This in-person event will celebrate 10 years of research from the Joint Center… Read More
-
A Message from JCESR: In Memory of George Crabtree
It is with heavy hearts that we say goodbye to George Crabtree, a Senior Scientist and Distinguished Fellow at Argonne National Laboratory, and Director of the Joint Center for Energy Storage Research (JCESR), who passed away unexpectedly on January 23. Dr. Read More
-
Cyanopyridines As Extremely Low-Reduction-Potential Anolytes for Nonaqueous Redox Flow Batteries
Discovery of a cyanophenylpyridine derivative with a very low reduction potential and good stability during cycling. Read More
-
Characterizing Redoxmer – Electrode Kinetics Using a SECM-Based Spot Analysis Method
Identified asymmetries in electron transfer (ET) kinetics between the reduction and oxidation of ferrocene-based redoxmers by measuring the ET rate constants (kf/kb) as a function of electrode potential. Read More
-
Benzotriazoles as Low Potential Anolytes for Non-Aqueous Redox Flow Batteries
We developed an easy-to-synthesize benzotriazole-based anolyte with a high energy redox potential (-2.3 V vs Fc/Fc+) and high solubility that demonstrates stable electrochemical cycling performance. Read More

