Research Highlights
-
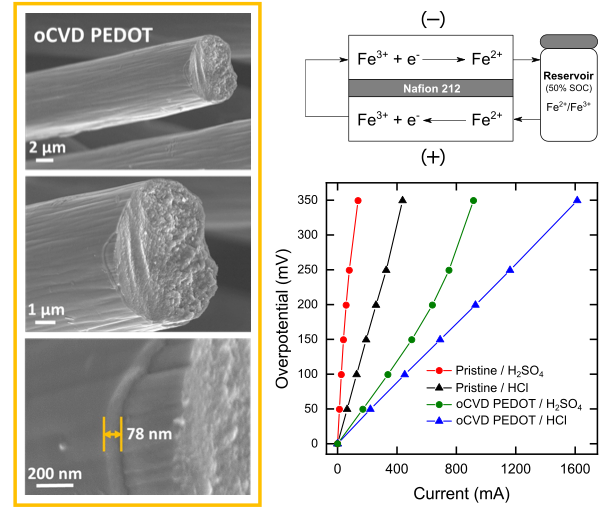
Ultrathin Conformal oCVD PEDOT Coatings on Carbon Electrodes Enable Improved Performance of Redox Flow Batteries
An oxidative chemical vapor deposition (oCVD) method is developed to deposit conformal, nanometric coatings on geometrically complex, porous carbon electrodes. Evaluation in a single electrolyte flow cell with an aqueous iron redox couple showed reduced kinetic, ohmic, and mass transport resistance as compared to uncoated samples. Read More
-
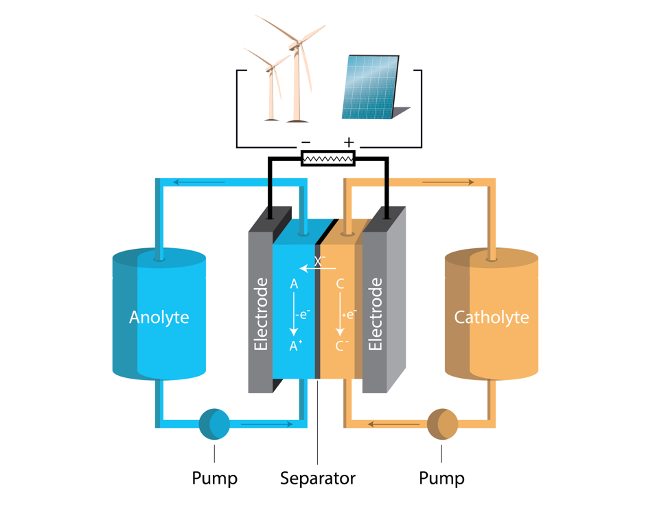
A Review of Electrochemical Advances in Non-Aqueous Organic Redox Flow Batteries
Advances in electrolytes for non-aqueous redox flow batteries and computational tools for their discovery and prediction are reviewed and explored. Read More
-
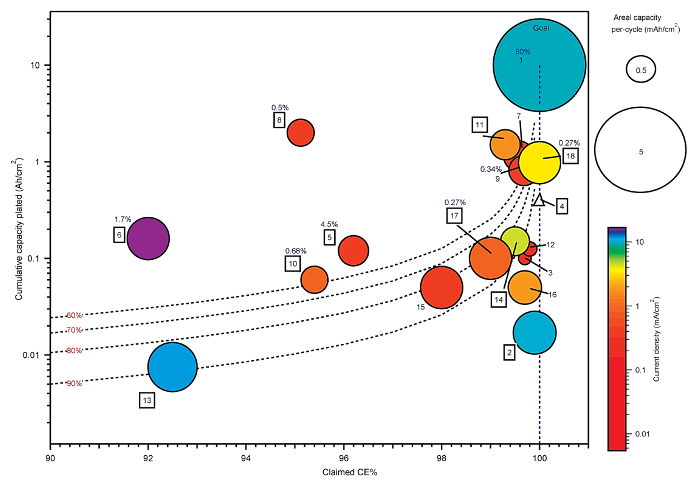
Realizing high zinc reversibility in rechargeable batteries
The first comprehensive comparison of the ~20 published studies evaluating Zn metal reversibility for rechargeable batteries shows significant variability in published test methods and parameters, and poor alignment toward commercial targets for zinc metal batteries. Significance and Impact Read More
-
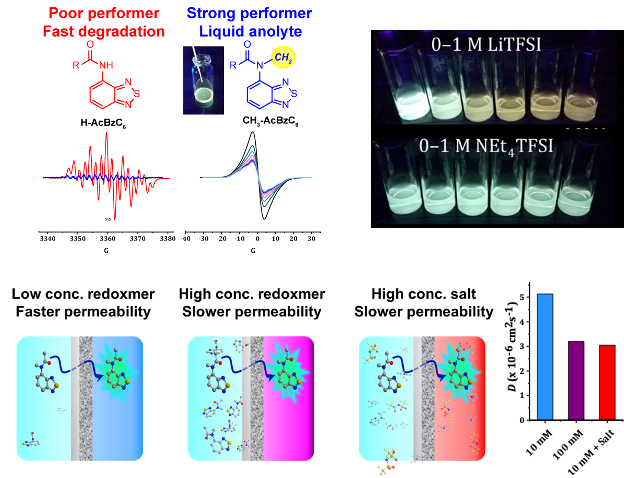
Seeing Battery Solutions Move to Assess the Health of Batteries
Organic redox-active molecules (redoxmers) are popular for flow battery fluids. Their structural variability comes with other properties that may be tailored for function. We discovered that fluorescence emission can be engineered into redoxmers, which allows real-time tracing of species crossover in a liquid flow cell, a key state of health (SOH) metric. The extracted permeability values show dependence on composition of electrolytes such as salt or redoxmer concentrations. Read More
-
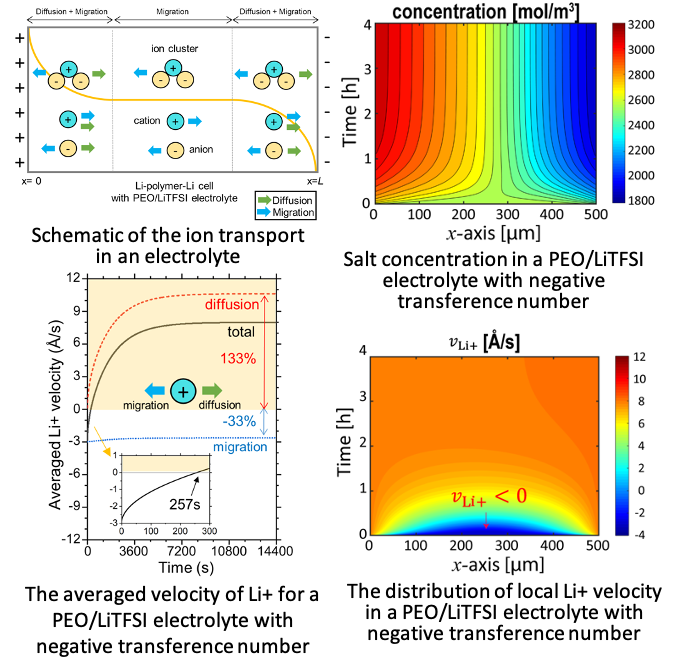
Continuum description of the role of negative transference numbers on ion motion in polymer electrolytes
This theoretical study not only reveals time and space dependence of ion motion in the PEO/LiTFSI electrolyte having negative transference number, but also provide guidance to experimentalists seeking to detect the direction of ion motion in these electrolytes. Read More
-
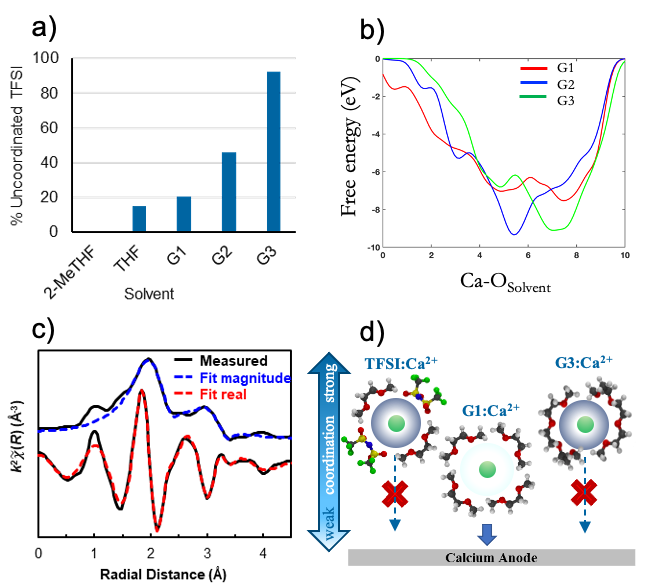
Influence of Ether Solvent and Anion Coordination on Electrochemical Behavior in Calcium Battery Electrolytes
Ethereal solvent structure determines Ca2+ solvation structure and the efficiency of calcium metal plating. In a moderate-coordinating solvent such as G1, anion coordination determines whether or not reversible plating takes place while in a strongly coordinating solvent such as G3, calcium plating is frustrated by a rigid solvation shell, regardless of anion. Read More
-
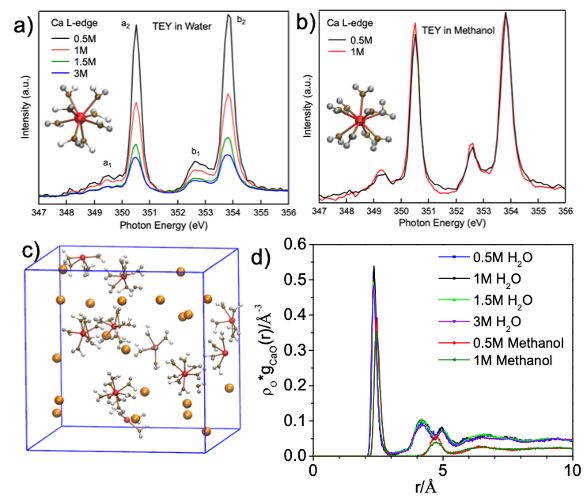
Probing calcium solvation by XAS, MD and DFT calculations
The solvation shell structures of Ca2+ in aqueous and organic solutions are probed by calcium L-edge soft X-ray absorption spectroscopy (XAS) and DFT/MD simulations. Read More
-
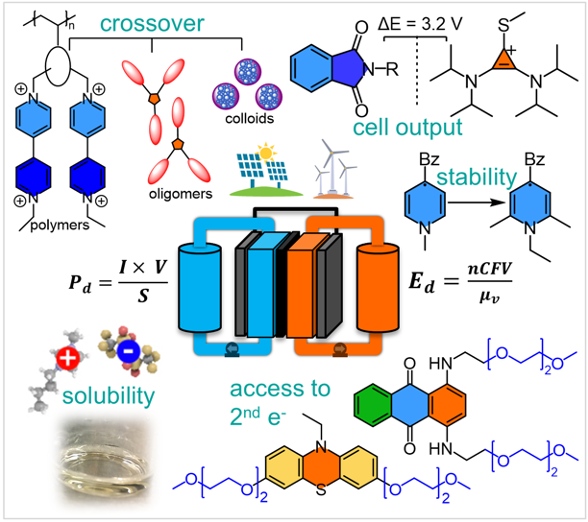
Recent Advancements in Rational Design of Non-Aqueous Organic Redox Flow Batteries
Organic redox-active molecules are considered as next-generation materials for non-aqueous redox-flow batteries (NAqORFBs). In this review, we screen the state-of-art advancements made in NAqORFBs, shed light upon the approaches that lie behind the developments, and detail the areas of particular interest through highlighted examples. Read More
-
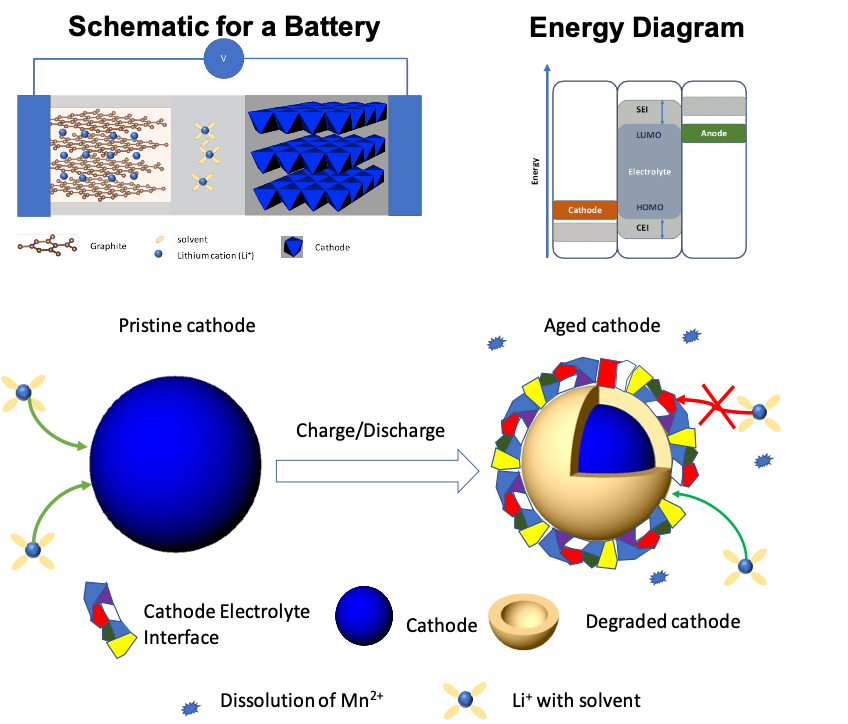
Electrolytes and Additives for Batteries Part I: Fundamentals and Insights on Cathode Degradation Mechanisms
This part I review serves as a self-contained article that addresses the fundamentals of batteries and cathode degradation mechanisms. Read More
-
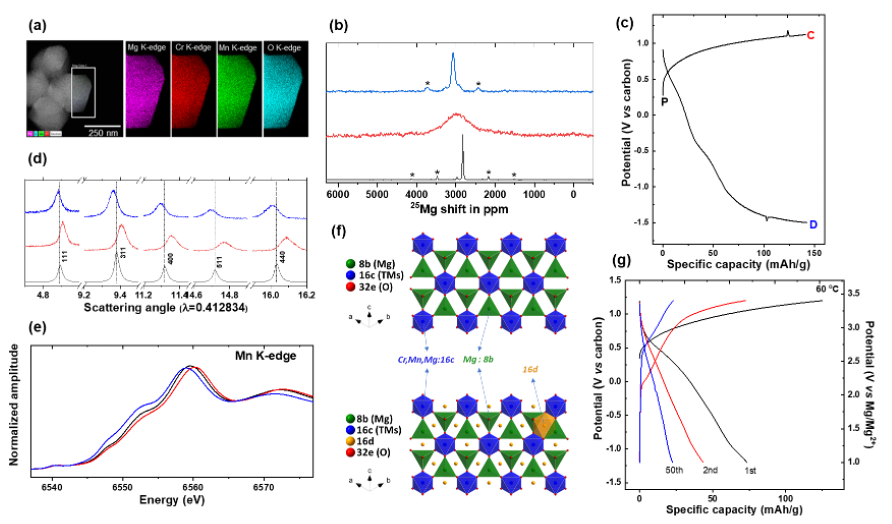
A High Voltage Mg-ion Battery Cathode via a Solid Solution Cr-Mn Spinel Oxide
The capability of the tailored MgCrMnO4 spinel to (de)intercalate Mg2+ electrochemically at high potentials was evaluated by the theoretical and experimental approaches. High Mg2+ activity was observed in bulk, with a remarkable degree of lattice breathing and reversibility. Read More
Latest Updates
-
You’re Invited - JCESR and Beyond: Translating the Basic Science of Batteries
Please join us at Argonne National Laboratory on Tuesday, April 4, 2023 for JCESR and Beyond: Translating the Basic Science of Batteries. Registration is now open. This in-person event will celebrate 10 years of research from the Joint Center… Read More
-
A Message from JCESR: In Memory of George Crabtree
It is with heavy hearts that we say goodbye to George Crabtree, a Senior Scientist and Distinguished Fellow at Argonne National Laboratory, and Director of the Joint Center for Energy Storage Research (JCESR), who passed away unexpectedly on January 23. Dr. Read More
-
Cyanopyridines As Extremely Low-Reduction-Potential Anolytes for Nonaqueous Redox Flow Batteries
Discovery of a cyanophenylpyridine derivative with a very low reduction potential and good stability during cycling. Read More
-
Characterizing Redoxmer – Electrode Kinetics Using a SECM-Based Spot Analysis Method
Identified asymmetries in electron transfer (ET) kinetics between the reduction and oxidation of ferrocene-based redoxmers by measuring the ET rate constants (kf/kb) as a function of electrode potential. Read More
-
Benzotriazoles as Low Potential Anolytes for Non-Aqueous Redox Flow Batteries
We developed an easy-to-synthesize benzotriazole-based anolyte with a high energy redox potential (-2.3 V vs Fc/Fc+) and high solubility that demonstrates stable electrochemical cycling performance. Read More

