Scientific Achievement
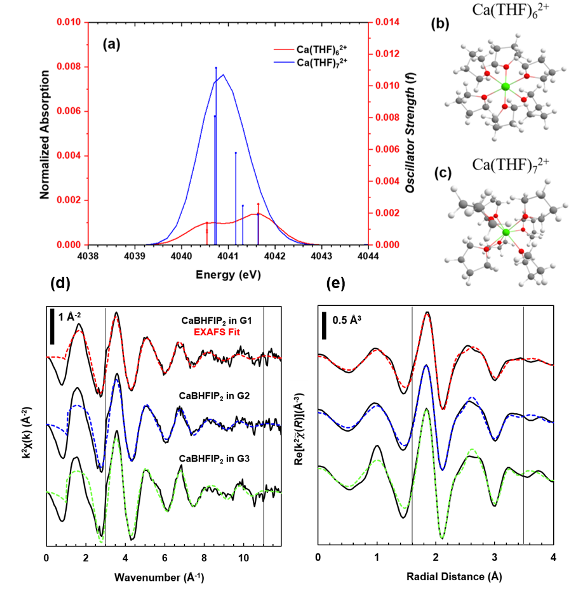
Through the combination of X-ray absorption fine structure (XAFS) and time-dependent density functional theory (TDDFT), descriptive measures of the local geometry, coordination, and electronic structure of Ca–ethereal complexes provide distinct structural trends depending on the extent of the Ca2+–solvent interaction.
Significance and Impact
We correlate these findings with electrochemical measurements of calcium tetrakis(hexafluoroisopropoxy)borate salts dissolved within ethereal solvents to provide insight into the preferred structural configuration of Ca2+ electrolytic solutions for optimized electrochemical plating and stripping.
Research Details
- X-ray absorption near-edge spectroscopy (XANES) studies at the Ca K-edge reveal a double peak pre-edge for Ca coordinated to 6 tetrahydrofuran (THF) ligands and was confirmed through TDDFT simulations of Ca–THF clusters.
- Extended X-ray absorption fine structures (EXAFS) measurements of multiple Ca-glyme solvation environments revealed an increase in ordering of the oxygen and carbon atoms around the Ca2+ cation with an increase glyme length (monoglyme < diglyme < triglyme). These changes are attributed to a more stable and rigid interaction of the larger glyme with Ca2+.

