Scientific Achievement
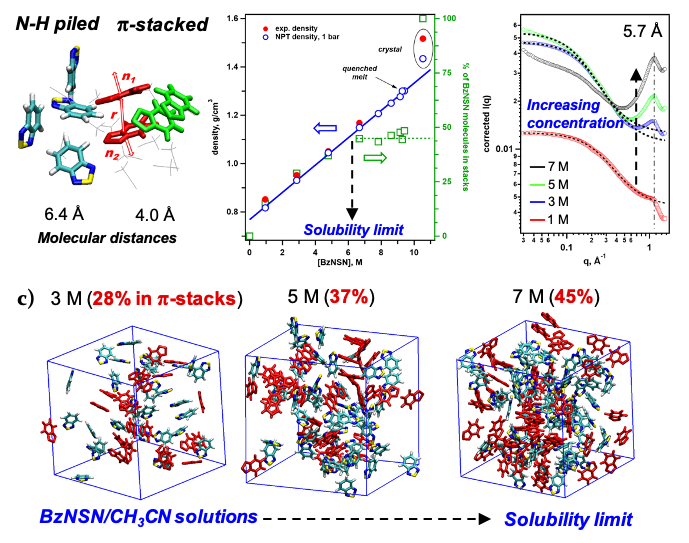
Organic redox-active molecules (redoxmers) are charge carriers in redox flow cells. Since the energy density of a battery fluid is proportional to concentration of active molecules, high molecular solubility is desirable. However, as the redoxmer solutions become crowded, solute-solute interactions become stronger, opposing high solubility. Here we demonstrate how a small redoxmer molecule, 2,1,3-benzothiadiazole (BzNSN), can achieve exceptionally high solubility through a competition of two packing motifs – N-H bond piling and π-stacking – that introduces disorder that frustrates crystallization in concentrated solutions.
Significance and Impact
Our observation that competing packing motifs improve redoxmer solubility suggests new strategies for designing concentrated all-organic electrolytes for redox flow cells.
Research Detail
- High BzNSN solubility in acetonitrile arises from competitive molecular interactions of forming face-to-face planar π-stacks or random bonds between N and H atoms in the molecule (N-H piling)
- Small-angle X-ray scattering (SAXS) indicates these two solute-solute interactions in close competition
- The solubility limit of ~6 M corresponds to a leveling off of the fraction of molecules in π-stacks
- Molecular dynamics reveals details of molecular interactions and their effect on solution properties

