Scientific Achievement
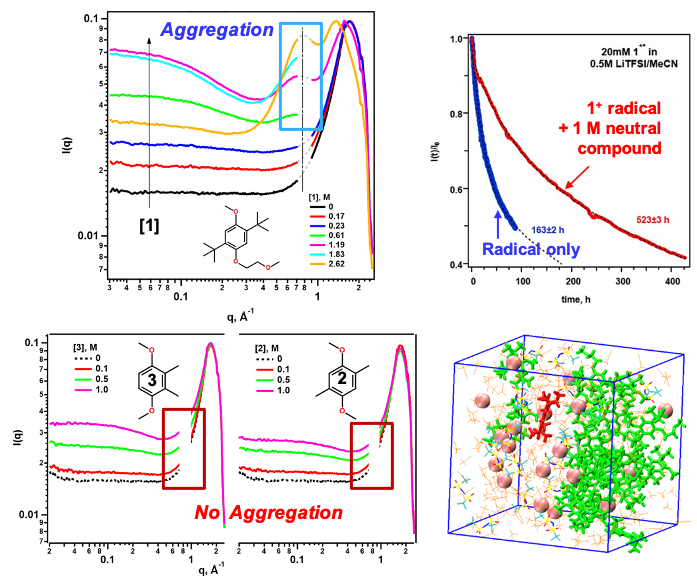
Structural diversity of organic redox-active molecules (redoxmers) permits tuning not only solute-electrolyte interactions but also solute-solute interactions in concentrated electrolyte solutions. The 1,4-dimethoxybenzene (DMB) family is an example of such flexibility. We show that DMB molecule 1 forms extended aggregates in concentrated solutions whereas simpler DMB molecules 2 and 3 do not. The resulting networks expel solvent and aggregated ions to small and increasingly isolated nanodomains. Charged redoxmer molecules are also expelled to such domains and their stability increases dramatically due to nanoconfinement (“microreactor”) effects.
Significance and Impact
Stability of organic redoxmers in their charged states is paramount for continuous battery operation. The formation of extended molecular networks in concentrated solutions of some redoxmers and nanoconfinement of their charged states is a novel strategy for improving chemical stability.
Research Detail
- Small-angle X-ray scattering shows redoxmer 1 aggregates in concentrated solutions, which are not seen for 2 and 3
- Molecular dynamics simulation shows that above 0.5-1 M, 1 forms a continuous contact network with dispersed solvent/ion domains
- The radical cation of 1 is 3X more stable relative to dilute solutions when confined in such nanodomains

