Scientific Achievement
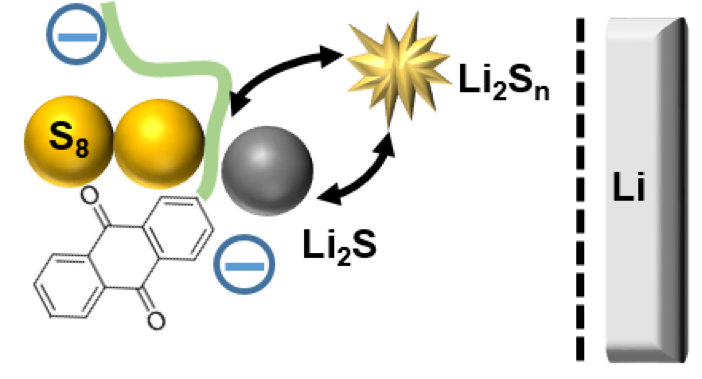
We showed that the incorporation of an all-organic redox-active polymer 2,6-polyanthraquinone (PAQ) into the cathode improves capacity retention in galvanostatic cycling and inhibits Li corrosion and S deposition.
Redox reactions of this polymer are shown to be strongly coupled to S redox cycle.
Significance and Impact
Complex nanoengineered cathodes required to suppress the unwanted polysulfide shuttling in lithium-sulfur batteries hinder detailed understanding of their function, impeding the development of new materials.
We showed that a rationally chosen organic polymer can suppress polysulfide shuttling, and its function can be elucidated in detail.
Research Details
- Galvanostatic cycling shows improvement in capacity retention in the presence of PAQ.
- SEM/EDS and XPS shows significantly reduced Li corrosion and S deposition.
- CV, EPR and IR suggest that the electrochemical reactions of PAQ form Li[PAQ] that mediates the S redox cycle.

