Scientific Achievement
Identification and understanding of cycle life limiting factors of Li-S batteries under lean electrolyte conditions; Identification of a NH4TFSI additive to effectively mitigate the uncontrollable passivation issue arising from accumulation of insulating Li2S.
Significance and Impact
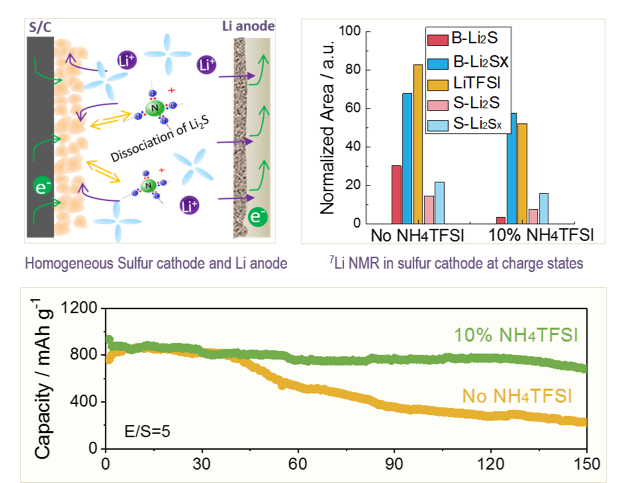
NH4TFSI additive enhances the dissociation of Li2S, and largely reduces the insoluble and insulating Li2S particles in sulfur cathodes, which facilitates reversible and sustainable redox reactions and significantly improves the cycle life of a Li-S battery under lean electrolyte conditions.
Research Details
- The cycle life of Li-S batteries under lean electrolyte condition largely depends on the electrolyte to sulfur ratio (E/S). SEM, NMR and XPS measurements indicate that a low E/S ratio creates a critical passivation issue resulting from uncontrollable and irreversible accumulation of Li2S.
- The NH4TFSI additive increases the solubility of Li2S and other short-chain species effectively by tailoring the ionic strength of the solution and promoting Li2S dissociation.
SEM and EIS measurement indicate that NH4TFSI enables a homogeneous sulfur cathode and Li anode morphology with sustainable reaction interfaces upon cycling under lean electrolyte conditions.

