Scientific Achievement
Observation of molecular layering and insight into Li-ion salt concentration dependence of molecular orientation at metal-oxide electrolyte interfaces relevant to Li-ion batteries.
Significance and Impact
Detailed understanding of the electrolyte-electrode interface and electric double layer in Li-ion batteries will inform strategies to mitigate capacity losses associated with interfaces and can help improve charging times in batteries.
Next steps are charged interfaces – understand how electric field controls molecular organization (electrical double layer) and the initial formation and subsequent growth of the solid-electrolyte interface layer (reaction of electrolyte).
Research Details
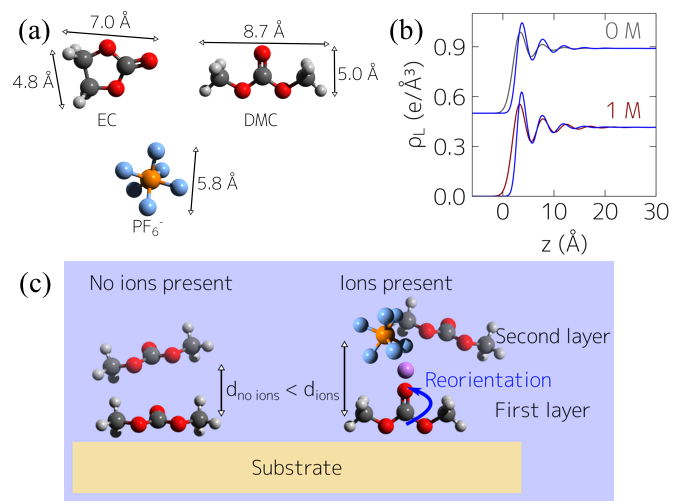
- Combined interface sensitive X-ray reflectivity (XRR) with molecular dynamics (MD) simulations to gain insight into the sapphire interface with Li-ion battery liquid electrolytes and found good agreement between experiment and simulation.
- Observed layering of electrolyte molecules with molecules in the first interfacial layer aligned parallel to the interface.
- Investigated how varying lithium hexafluorophosphate (LiPF6) salt concentrations in ethylene carbonate (EC) and dimethyl carbonate (DMC) impacted interfacial molecular organization.
- With increasing LiPF6 concentration, found a reorientation of solvent molecules in the presence of ions.

