Scientific Achievement
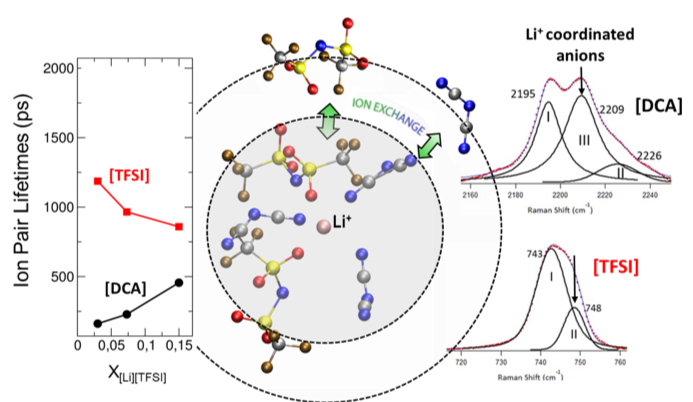
The local structure of Li+ in this eutectic is found to be heterogenous and preferentially solvated by [DCA], which is related to the transport properties.
Significance and Impact
The results show that the solvation and transport properties of charge carriers in ILs can be modified via the presence of multiple ions with varying degree of coordination, which provides an approach to impact the performance in electrochemical processes.
Research Details
- The structural and dynamical changes in the solvation shell surrounding Li+ in a multi-anion environment are studied by Raman spectroscopy and molecular dynamics (MD) simulations.
- A 1:9 volumetric mixture of [PYR13][TFSI]:[EMIM][DCA] formed an eutectic that exhibited a lower melting point than that of either parent IL.
- The calculated decrease in ion pair lifetime of [TFSI] with Li+ at higher concentrations of the lithium salt is believed to depress the decrease in conductivity with increasing [Li][TFSI] concentration.

