Scientific Achievement
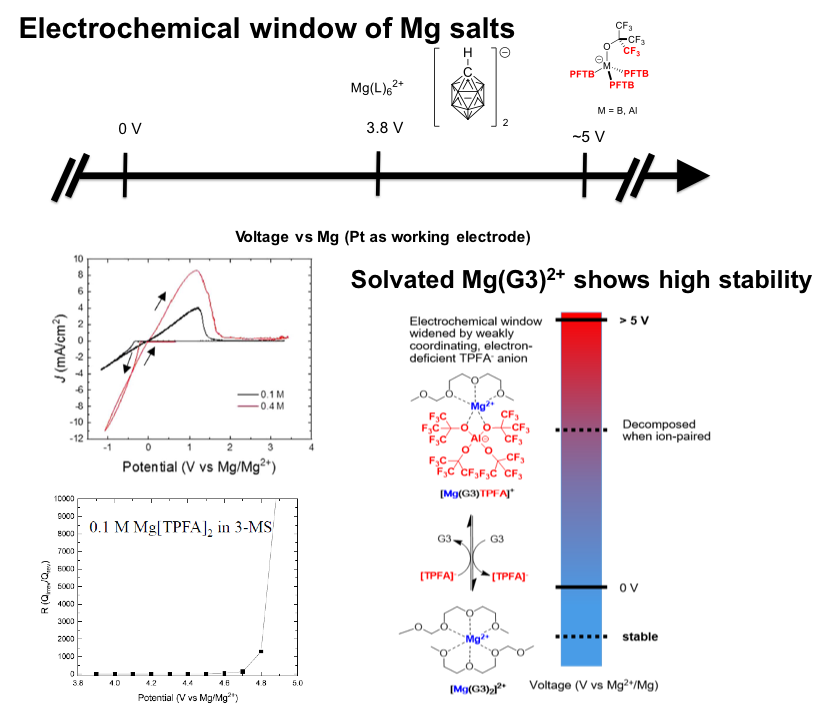
Mg[Al{OC(CF3)3}4]2 (Mg[TPFA]2 ) with perfluorinated, e- deficient anions ([TPFA]−) possesses high thermodynamic oxidative stability with high coulombic efficiency for Mg plating/stripping.
Significance and Impact
The electrochemical window of Mg salt was widened to 4.9 V vs Mg/Mg2+ using a synthetic approach: t-butyl perfluorinated aluminate anion was first demonstrated in a MV salts. The weakly associating concept was adapted to increase thermodynamic stability of anions by decreasing the propensity of ion pair formation and consequent charge transfer decomposition.
Research Details
- Alcoholysis of LiAlH4 with (CF3)3COH followed by Li/H cation exchange to generate H[TPFA], which later reacts with Mg(HMDS)2 gives a pure salt of Mg[TPFA]2.
- Computational and electrochemical analyses suggested a mitigated cathodic decomposition as well as enhanced anodic stability from electron-withdrawing CF3 groups.
- Detailed NMR, IR and SEM/EDX further support that the weak coordination to Mg2+ in solution is important for maintaining the wide electrochemical window of Mg salt.

