Scientific Achievement
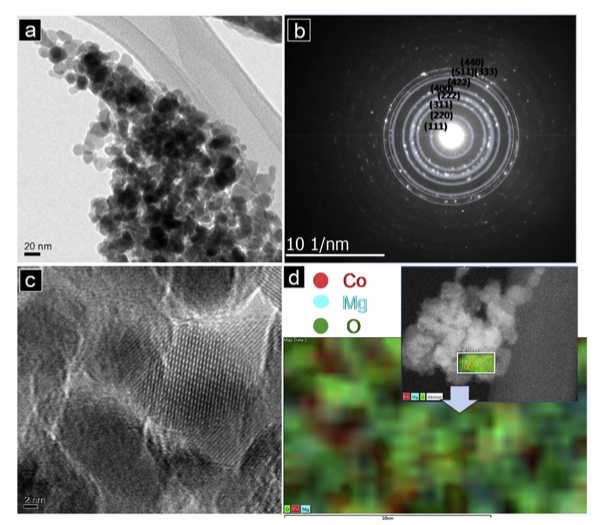
We have been exploring the versatility and mechanism of Mg insertion into several transition metal spinel compounds and correlating the results with electron count. In this publication we established that the MgCo2O4 works by a conversion mechanism – MgO extraction and precipitation as the primary electrochemical activity with loss of oxide and reduction of cobalt. System characterization was complicated by the observed solid solution between MgCo2O4 – Co3O4 but overcome by utilizing a variety of techniques to fully characterize the materials synthesized.
Significance and Impact
We have identified and confirmed MgCo2O4 as a conversion cathode in the Mg-Ion electrochemical systems due to the high mobility of d7 Co+2 in the host lattice between the two cation sites.
Research Details
- Amorphous MgO was observed by numerous techniques at the electrochemical interface between the cathode and the electrolyte, indicative of a conversion mechanism.
- The presence of de-magnesiated cobalt oxides was confirmed by multiple techniques as the primary by-product of the magnesium oxide formation. Reactions of the short-lived metastable charged cathode with electrolyte solvents were considered as a possible pathway for the reaction.

