Multivalent Intercalation
-
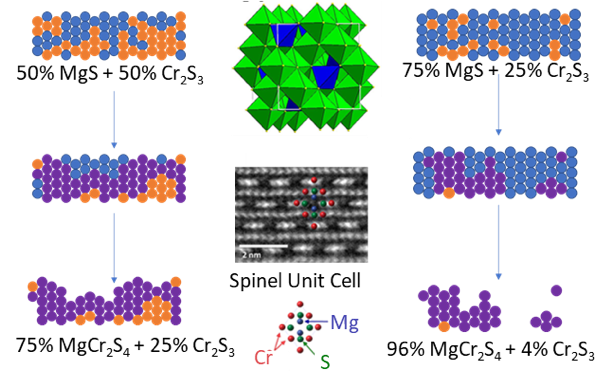
Synthesis of MgCr2S4 Thiospinel as a Potential Magnesium Cathode
MgCr2S4 was synthesized for the first time. This thiospinel material is predicted to have a high insertion voltage relative to the isostructural MgTi2S4 studied by the center. This synthesis serves as a starting point for making other unknown battery materials. Read More
-
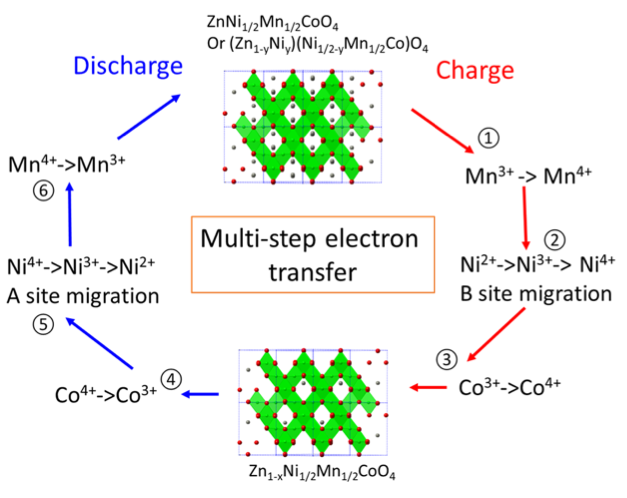
ZnNixMnxCo2–2xO4 Spinel as a High-Capacity Cathode Material for Nonaqueous Zn-Ion Batteries
A new series of spinels, ZnNixMnxCo2-2xO4, are reported as a high-voltage and high-capacity cathode material for non-aqueous Zn-ion batteries. Mn, Ni co-substitution for Co in ZnCo2O4 is demonstrated to be an efficient method to facilitate Zn-deintercalation and enhance discharge capacity, which provides guidelines for designing more attractive multivalent cathodes materials. Read More
-
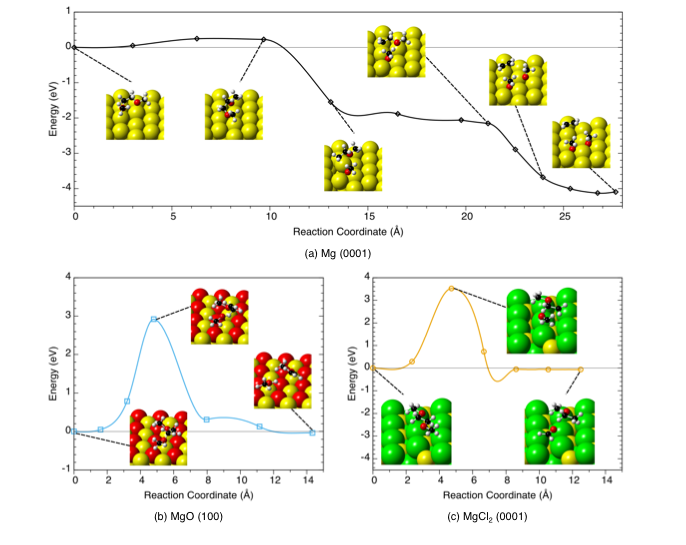
Reaction Pathways for Solvent Decomposition on Magnesium Anodes
Dimethoxyethane (DME) is predicted to rapidly decompose to ethylene gas and other products on the metallic Mg surface, whereas the presence of an oxide or chloride surface film on a Mg anode is predicted to limit solvent decomposition. Read More
-
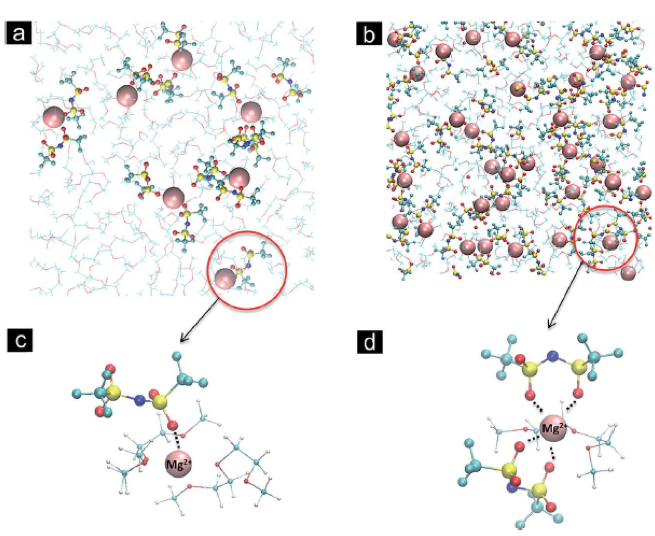
Elucidating Solvation Structures for Rational Design of Multivalent Electrolytes—A Review
A review of molecular-level interactions governing multivalent electrolyte behavior is presented. The current understanding of solvation structure, stability and transport properties of electrolytes for secondary Mg, Zn and Ca batteries is critically examined from experimental and theoretical studies. Read More
-
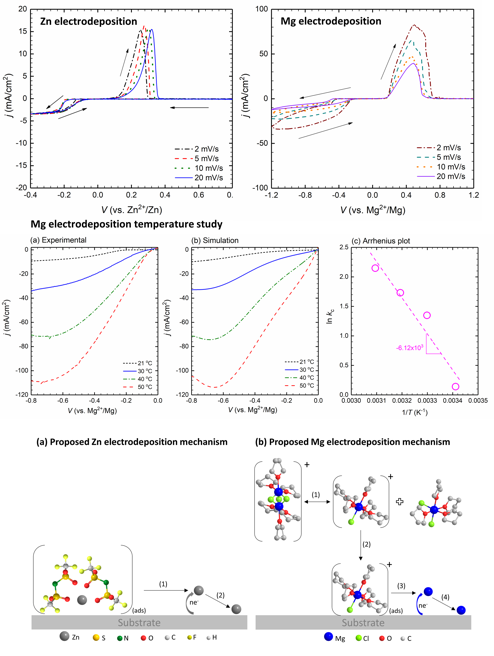
Elucidating Zn and Mg Electrodeposition Mechanisms in Nonaqueous Electrolytes for Next-Generation Metal Batteries
The development of nonaqeuous electrolytes enabling reversible and efficient deposition/stripping of multivalent metals has been hindered because of the complexity of electrolyte properties and behaviors. Different cations exhibit different deposition efficacy. Our research explores Zn and Mg deposition mechanisms and explains differences between the two cations. Read More
-
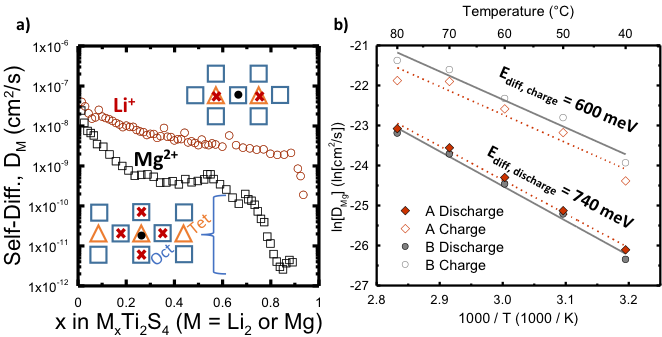
Mono vs Divalent Cation Diffusion in Thiospinel Ti2S4
We showed why the positive electrode material, MgxTi2S4, does not work well at room temperature, unlike Li2xTi2S4, which works very well. Furthermore, we confirmed that theoretical diffusion calculations predict accurate results at low x values. Read More
-
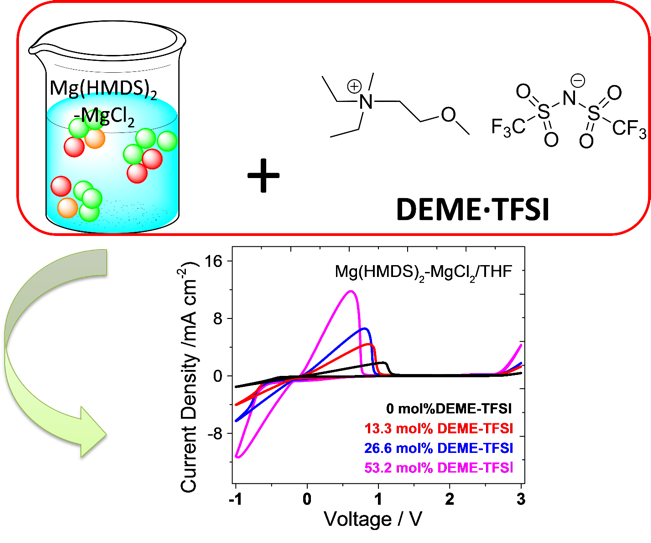
Ionic Liquid as an Effective Additive for Rechargeable Magnesium Batteries
The effect of the addition of an ionic liquid DEME·TFSI to an electrolyte solution of Mg(HMDS)2-MgCl2 in THF was spectroscopically and electrochemically studied. Reversible magnesium deposition/dissolution and cycling of Mg-Mo6S8 coin cells were achieved with the DEME·TFSI-modified magnesium electrolyte. Read More
-
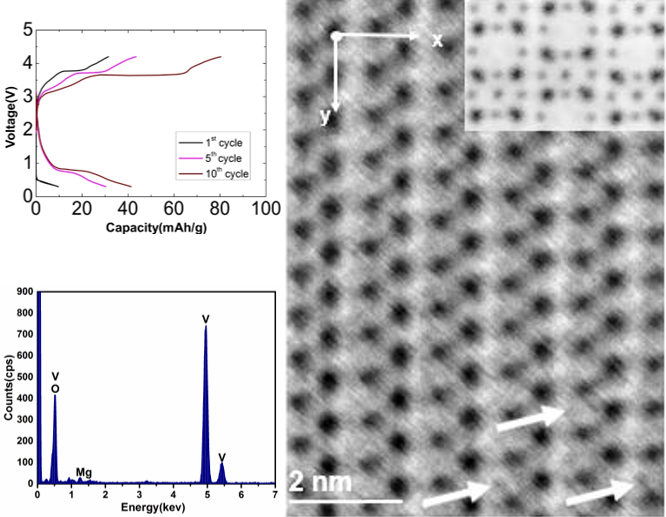
Investigation of Mg Intercalation into α-V2O5 Using Atomic Resolution Transmission Electron Microscopy
We examine the effects of Mg intercalation into α-V2O5 using atomic-resolution scanning transmission electron microscopy (STEM) and electron energy-loss spectroscopy. The position of intercalated Mg within the V2O5 unit cell is directly identified using annular bright-field imaging, and found to be in agreement with prior density-functional theory models. Read More
-
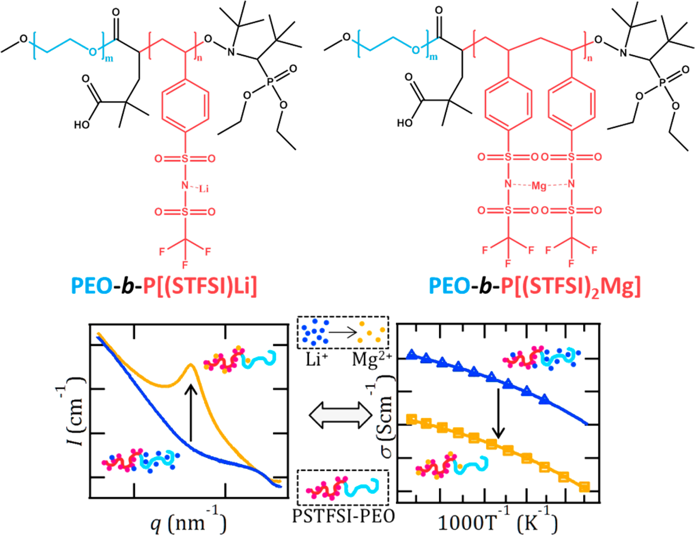
Interaction Parameter (χ) and Ion Dissociation in Lithium and Magnesium Single-Ion-Conducting Block Copolymers Electrolytes
Ion dissociation and block copolymer thermodynamics are intimately coupled: ion dissociation in lithiated systems suppresses microphase separation. Read More
-
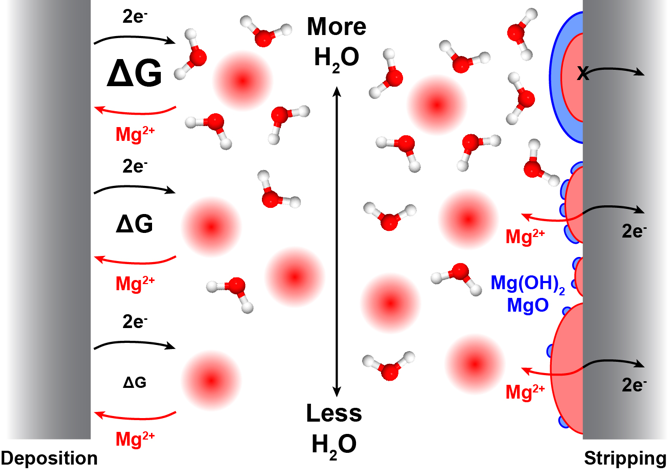
Impact of Trace Impurities on the Reversibility of Mg Anodes
Trace amounts of water (≤ 3 ppm) are shown to strongly influence both the kinetics of Mg deposition and the reversibility of Mg stripping, with Cl- mitigating passivation by H2O by strongly modifying the Mg surface chemistry. Read More
Latest Updates
-
You’re Invited - JCESR and Beyond: Translating the Basic Science of Batteries
Please join us at Argonne National Laboratory on Tuesday, April 4, 2023 for JCESR and Beyond: Translating the Basic Science of Batteries. Registration is now open. This in-person event will celebrate 10 years of research from the Joint Center… Read More
-
A Message from JCESR: In Memory of George Crabtree
It is with heavy hearts that we say goodbye to George Crabtree, a Senior Scientist and Distinguished Fellow at Argonne National Laboratory, and Director of the Joint Center for Energy Storage Research (JCESR), who passed away unexpectedly on January 23. Dr. Read More
-
Cyanopyridines As Extremely Low-Reduction-Potential Anolytes for Nonaqueous Redox Flow Batteries
Discovery of a cyanophenylpyridine derivative with a very low reduction potential and good stability during cycling. Read More
-
Characterizing Redoxmer – Electrode Kinetics Using a SECM-Based Spot Analysis Method
Identified asymmetries in electron transfer (ET) kinetics between the reduction and oxidation of ferrocene-based redoxmers by measuring the ET rate constants (kf/kb) as a function of electrode potential. Read More
-
Benzotriazoles as Low Potential Anolytes for Non-Aqueous Redox Flow Batteries
We developed an easy-to-synthesize benzotriazole-based anolyte with a high energy redox potential (-2.3 V vs Fc/Fc+) and high solubility that demonstrates stable electrochemical cycling performance. Read More

