Research Highlights
-
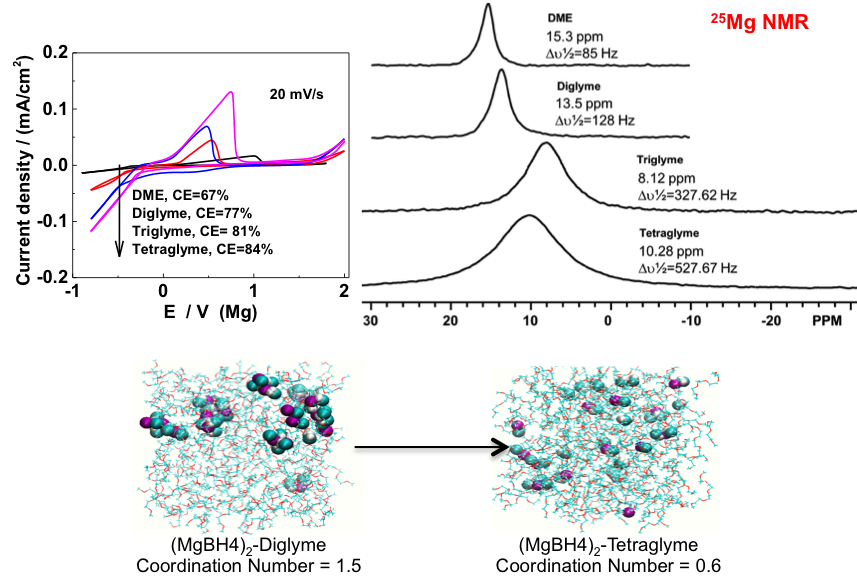
Designing Solvation in Magnesium Electrolytes
Multivalent salts are prone to ion-pair formation in many solvents, particularly the glymes that provide stability at metal anode Read More
-
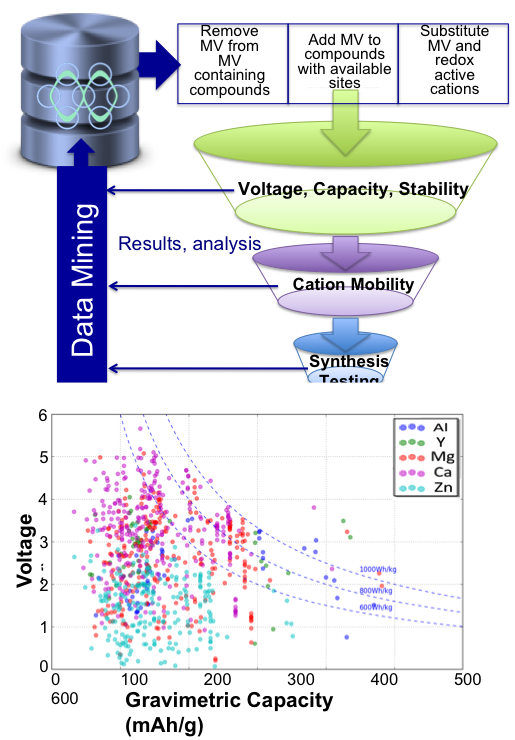
Screening of Novel Multivalent Cathodes
Developed strategies and software infrastructure for generation and design of multivalent intercalation candidates Read More
-
EDL: Discovering Electrochemistry at Atomic and Molecular Levels
Established in situ Electrochemical Discovery Lab (EDL) for systematic studies of wet and dry interfaces for materials-by-design and electrolytes-by-design at atomic and molecular levels Read More
-
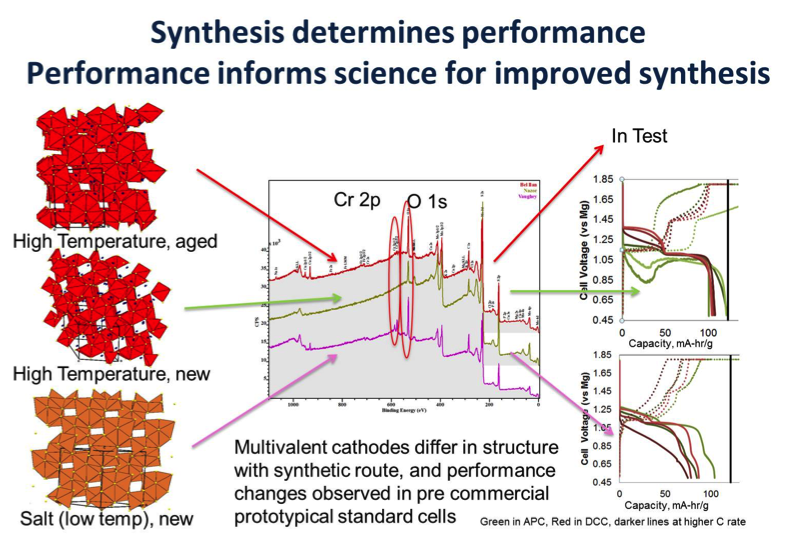
Prototyping and Performance of Mg/Mo6S8 Coin Cells
Achieved maximum theoretical capacity for the Mg2+/Mo6S8 system, demonstrated capability for rapid test and prototype of multiple cathodes and electrolyte. Read More
-
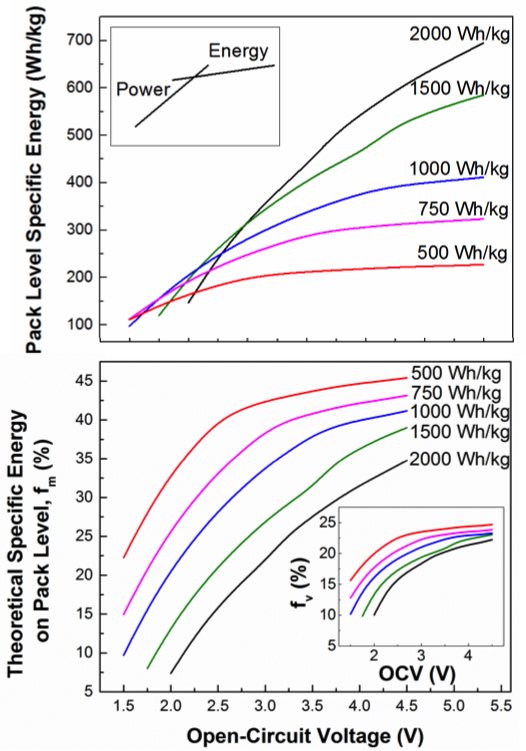
Fraction of Theoretical Specific Energy Achieved at Battery Pack Level Is Very Sensitive to the Cell Chemistry
Cell chemistry properties, rather than engineering/packaging approach, are the key factors in determining the fraction of battery material specific energy captured at pack level Read More
-
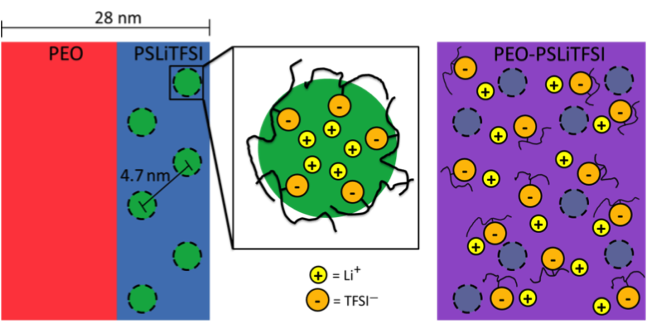
Morphology and Conductivity Relationship of Single-Ion-Conducting Block Copolymer Electrolytes
Showed that an order to disorder transition in PEO-PSLiTFSI is qualitatively different than that in typical block copolymers. At this transition, the ionic conductivity increased several orders of magnitude. Read More
-
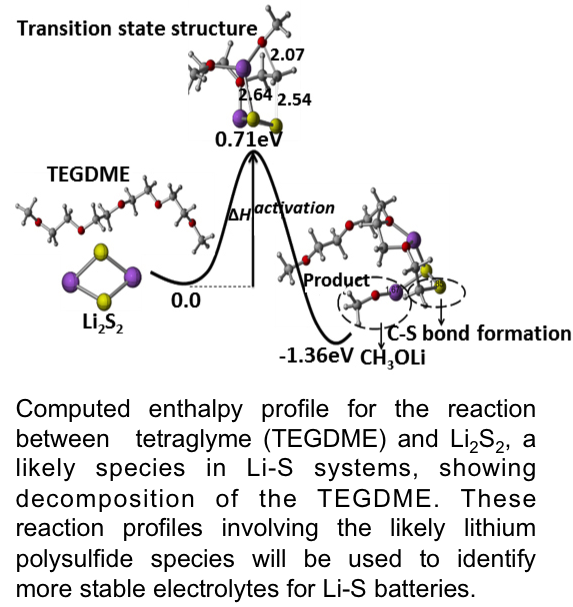
Predicting Reaction Sequences for Li-S Batteries
First comprehensive, high level and accurate quantum chemical predictions of (i) complex discharge reaction sequences that convert lithium (Li) and sulfur (S) to Li2S in Li-S batteries and (ii) harmful side reactions that consume the Li and S active components and break down the electrolyte. Read More
-
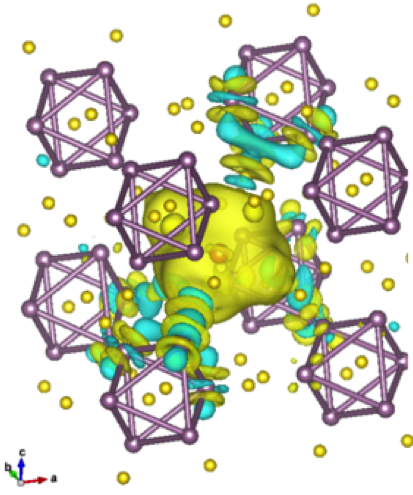
New Insights Into a Functioning Mg-ion Cathode–Chevrel Phase Mo6S8
First-principles analysis of the screening of Mg2+ ions upon intercalation within metallic Mo6S8 Read More
-
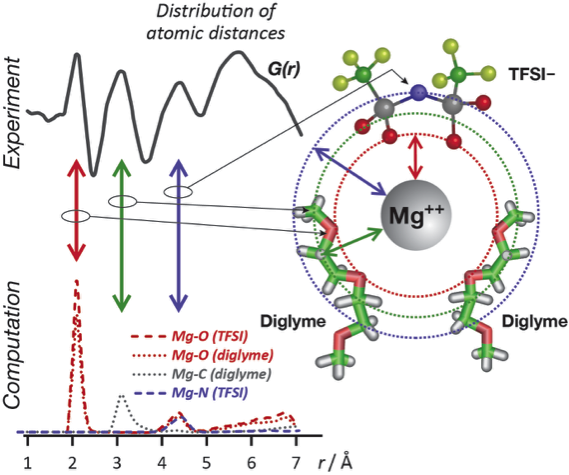
Mg++ Solvation Shell in Electrolyte for Multivalent Batteries
A new collaborative x-ray scattering and molecular dynamics simulation approach reveals the structure and energetics of Mg++ ion solvation in a diglyme electrolyte. Read More
-
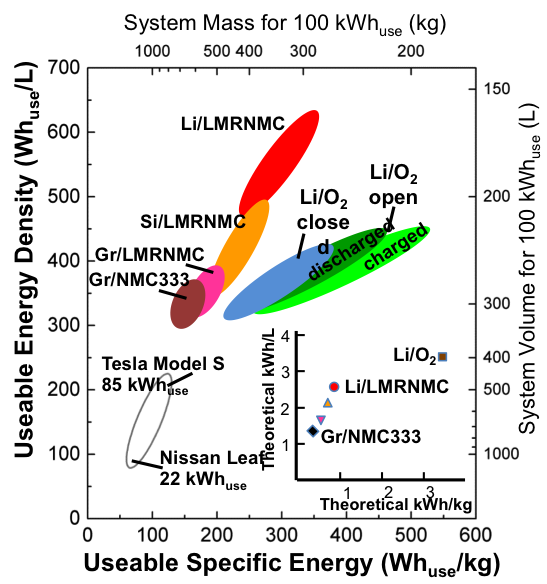
Quantifying the Promise of Lithium‒Air Batteries for Electric Vehicles
First comprehensive materials-to-system analysis of performance and manufacturing cost of Li-O2 batteries Read More
Latest Updates
-
You’re Invited - JCESR and Beyond: Translating the Basic Science of Batteries
Please join us at Argonne National Laboratory on Tuesday, April 4, 2023 for JCESR and Beyond: Translating the Basic Science of Batteries. Registration is now open. This in-person event will celebrate 10 years of research from the Joint Center… Read More
-
A Message from JCESR: In Memory of George Crabtree
It is with heavy hearts that we say goodbye to George Crabtree, a Senior Scientist and Distinguished Fellow at Argonne National Laboratory, and Director of the Joint Center for Energy Storage Research (JCESR), who passed away unexpectedly on January 23. Dr. Read More
-
Cyanopyridines As Extremely Low-Reduction-Potential Anolytes for Nonaqueous Redox Flow Batteries
Discovery of a cyanophenylpyridine derivative with a very low reduction potential and good stability during cycling. Read More
-
Characterizing Redoxmer – Electrode Kinetics Using a SECM-Based Spot Analysis Method
Identified asymmetries in electron transfer (ET) kinetics between the reduction and oxidation of ferrocene-based redoxmers by measuring the ET rate constants (kf/kb) as a function of electrode potential. Read More
-
Benzotriazoles as Low Potential Anolytes for Non-Aqueous Redox Flow Batteries
We developed an easy-to-synthesize benzotriazole-based anolyte with a high energy redox potential (-2.3 V vs Fc/Fc+) and high solubility that demonstrates stable electrochemical cycling performance. Read More

