Research Highlights
-
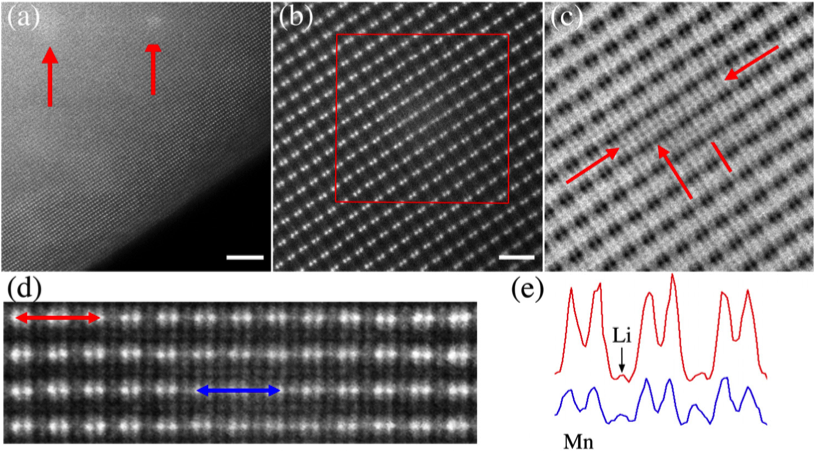
Understanding the Structural and Electronic Evolution of Li2MnO3 During Electron Irradiation Via Electron Microscopy
Electrochemical cycling induces irreversible changes to the oxide structure such as Li/O evolution and crystal rearrangements Read More
-
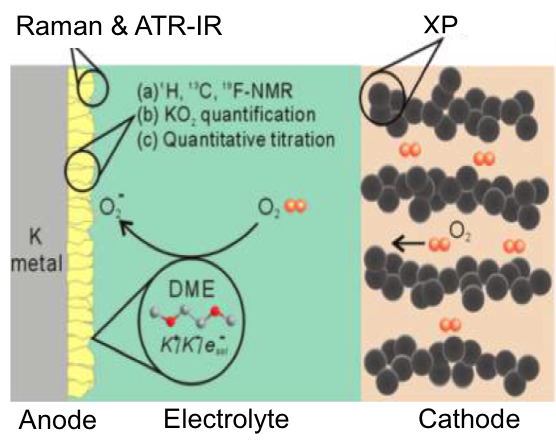
Understanding Side Reactions in K-O2 Batteries for Improved Cycle Life: a Combined DFT and Experimental Study
First comprehensive study of side reactions that dictate the fundamental electrochemistry of K-O2 battery has been carried out Read More
-
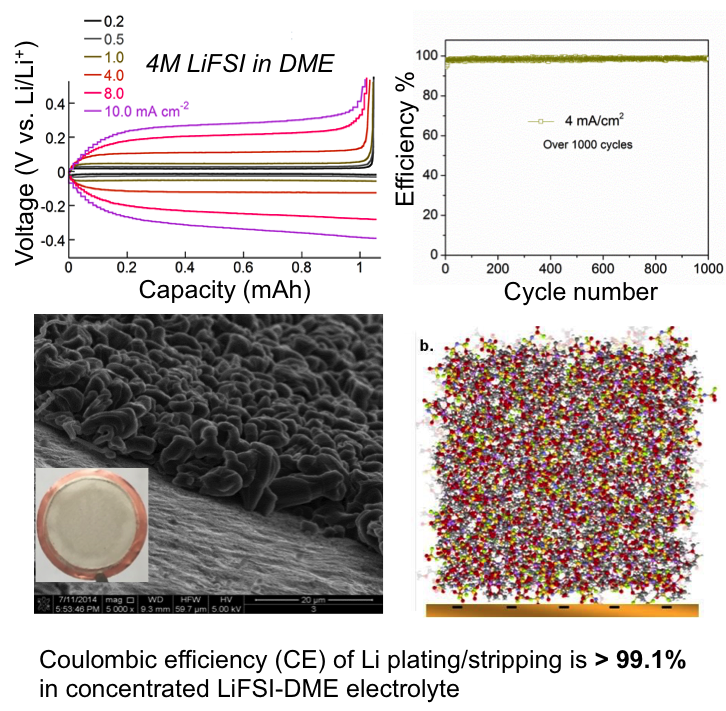
High Rate and Stable Cycling of Lithium Metal Anode
Lithium metal is an ideal battery anode. However, dendrite growth and limited CE during cycling have limited its practical applications. High CE (up to 99.1%) without dendrite growth is achieved by using highly concentrated electrolytes for lithium plating/stripping. Read More
-
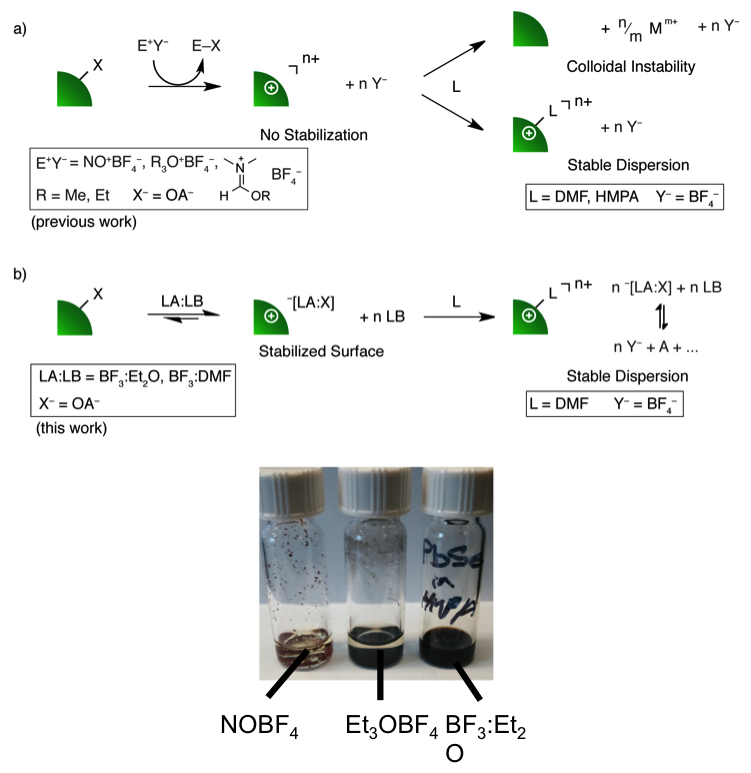
Mechanistic Insight into the Formation of Cationic Naked Nanocrystals Generated Under Equilibrium Control
Developed a class of ligand-stripping reagents that stabilize the nanocrystal (NC) surface during the stripping reaction, leading to improved dispersibility. Read More
-
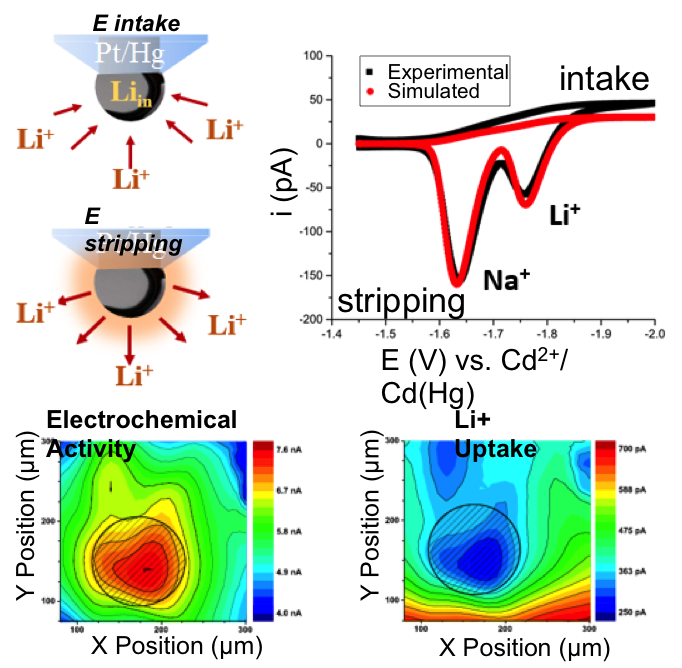
Imaging Heterogeneous Ion Transfer: Lithium Ion Quantification using Mercury Amalgams as In Situ Electrochemical Probes in Nonaqueous Media
Quantitative electrochemical probes for the in situ detection of reactive fluxes of alkaline ions in non-aqueous media were developed Read More
-
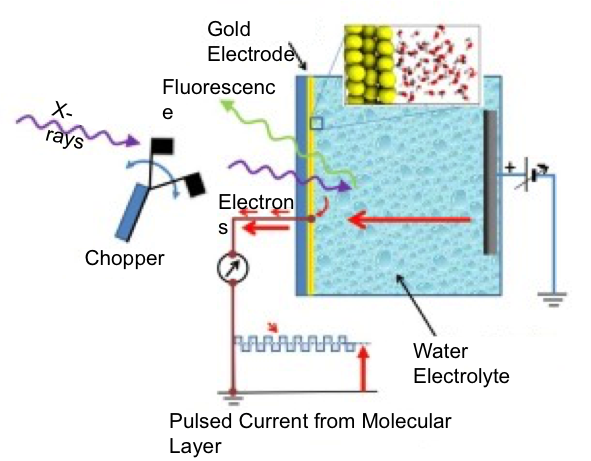
The Structure of Interfacial Water on Gold Electrodes Studied by X-ray Absorption Spectroscopy
First X-ray absorption measurement of electrolyte structure in vicinity of biased electrode Read More
-
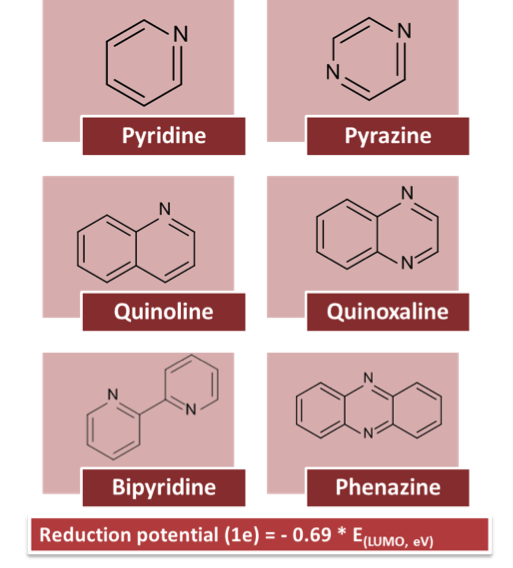
Predicting Electrochemical Windows of Nitrogen Containing Aromatic Molecules
A descriptive relationship is derived for computing reduction potentials of quinoxaline derivatives from the orbital energies of the neutral molecules without time-consuming free energy calculations Read More
-
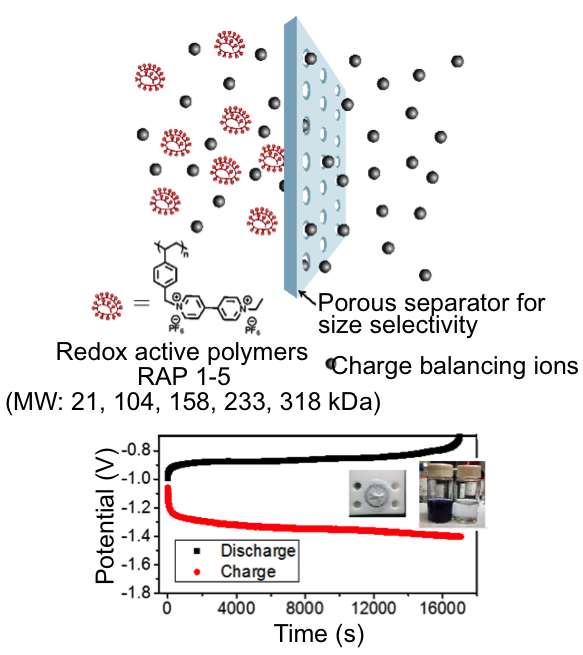
New concepts in Redox Flow: “Impact of Redox-Active Polymer Molecular Weight on the Electrochemical Properties and Transport Across Porous Separators in Nonaqueous Solvents”
Sized-based selective transport of supporting electrolyte across commercial Celgard porous separators is attainable by controlling the size of highly soluble (>2M) redox active polymers (RAPs) as storage material Read More
-
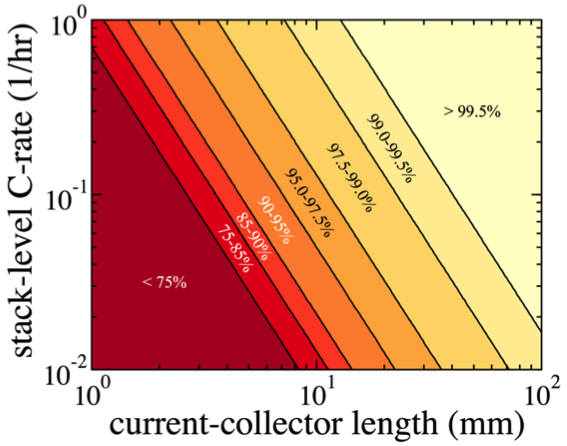
Electroactive-Zone Extension in Flow-Battery Stacks
Flowable suspensions that conduct both electrons and ions can enable the use of energy-dense electroactive species in flow batteries Read More
-
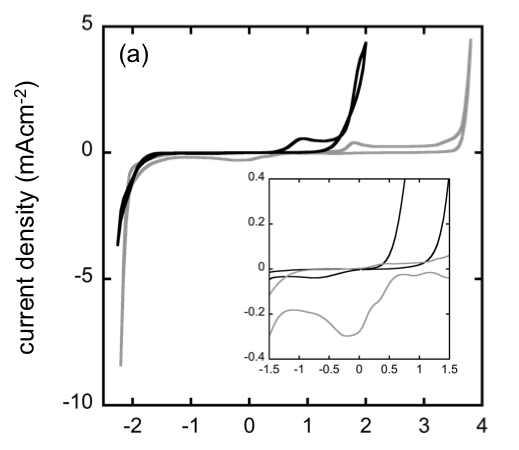
Electrochemistry of Magnesium Electrolytes in Ionic Liquids for Secondary Batteries
Ionic liquids (ILs) have wide electrochemical stability windows, low vapor pressures, and low flammabilities, making them attractive as replacements for conventional organic solvents like THF and DME Read More
Latest Updates
-
You’re Invited - JCESR and Beyond: Translating the Basic Science of Batteries
Please join us at Argonne National Laboratory on Tuesday, April 4, 2023 for JCESR and Beyond: Translating the Basic Science of Batteries. Registration is now open. This in-person event will celebrate 10 years of research from the Joint Center… Read More
-
A Message from JCESR: In Memory of George Crabtree
It is with heavy hearts that we say goodbye to George Crabtree, a Senior Scientist and Distinguished Fellow at Argonne National Laboratory, and Director of the Joint Center for Energy Storage Research (JCESR), who passed away unexpectedly on January 23. Dr. Read More
-
Cyanopyridines As Extremely Low-Reduction-Potential Anolytes for Nonaqueous Redox Flow Batteries
Discovery of a cyanophenylpyridine derivative with a very low reduction potential and good stability during cycling. Read More
-
Characterizing Redoxmer – Electrode Kinetics Using a SECM-Based Spot Analysis Method
Identified asymmetries in electron transfer (ET) kinetics between the reduction and oxidation of ferrocene-based redoxmers by measuring the ET rate constants (kf/kb) as a function of electrode potential. Read More
-
Benzotriazoles as Low Potential Anolytes for Non-Aqueous Redox Flow Batteries
We developed an easy-to-synthesize benzotriazole-based anolyte with a high energy redox potential (-2.3 V vs Fc/Fc+) and high solubility that demonstrates stable electrochemical cycling performance. Read More

