Scientific Achievement
First comprehensive, high level and accurate quantum chemical predictions of (i) complex discharge reaction sequences that convert lithium (Li) and sulfur (S) to Li2S in Li-S batteries and (ii) harmful side reactions that consume the Li and S active components and break down the electrolyte.
Significance and Impact
Identifying these reaction sequences allows intentional design of the highest performing, most stable and longest-lasting Li-S battery systems at the atomic and molecular level.
Research Details
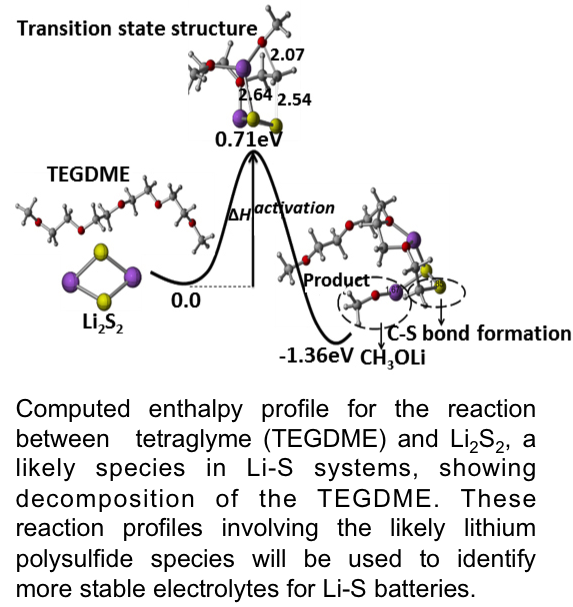
- The discharge reaction sequence in Li-S batteries is notoriously complex and poorly understood, with up to six or eight intermediate steps. This comprehensive quantum chemical analysis establishes a quantitative framework for analyzing, designing and achieving the best performance.
- This study identifies intermediate products that can break down the electrolyte needed to transport Li cations between the anode and cathode, removing essential materials needed in the Li-S battery.
- This fundamental knowledge is required for new electrolyte discovery to maximize the performance and minimize the degradation of Li-S batteries.
Work performed at Argonne National Laboratory, JCESR managing partner., and the University of Illinois R. S. Assary, L. A Curtiss, and J. S. Moore, Towards a Molecular Understanding of Energetics in Li-S Batteries Using Non-Aqueous Electrolytes: A High-Level Quantum Chemical Study J. Phys. Chem. C., 2014.

