Scientific Achievement
- A new collaborative x-ray scattering and molecular dynamics simulation approach reveals the structure and energetics of Mg++ ion solvation in a diglyme electrolyte.
Significance and Impact
- Solvation of the working ion in an electrolyte mediates critical phenomena including ion mobility, chemical reactions, solubility and ion transfer to electrodes. Understanding solvation behavior at the atomic scale is essential for future multivalent battery development.
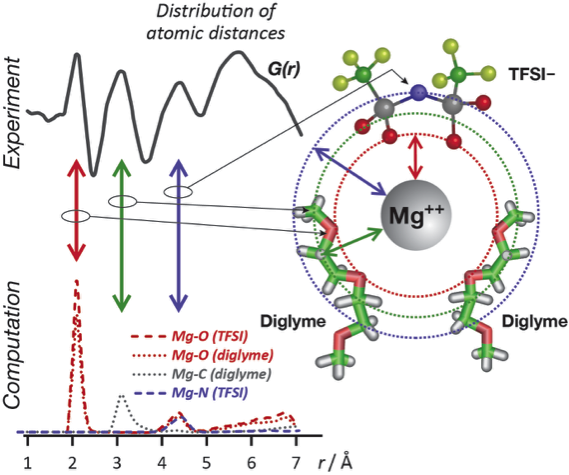
- A new experimental approach, multivariate analysis of the pair distribution function (PDF) derived from x-ray scattering, isolates the Mg++ solvation shell structure from the Mg-electrolyte mixture.
- Molecular dynamics simulations using parameters based on the experimental data interpret peaks in the PDF and provide high resolution, chemically-specific details not accessible experimentally.
- Location of TFSI- anions within the 1st solvation shell indicates that the Mg(TFSI)2 salt does not fully dissociate, with a less dynamic solvation structure than Li+, negatively affecting battery performance. Breaking strong Mg-anion pairs is a key design metric for future electrolytes.
Research Details
- X-ray total scattering and multivariate analysis of the pair distribution function performed at Argonne’s Advanced Photon Source.
- Molecular dynamics simulations of solvation shell structures and energies carried out at Lawrence Berkeley National Laboratory.
Work performed at Argonne National Laboratory and Lawrence Berkeley National Laboratory. Saul Lapidus, Nav Nidhi Rajput, Xiaohui Qu, Karena Chapman, Kristin Persson, Peter Chupas, Solvation structure and energetics of electrolytes for multivalent energy storage, Phys.Chem.Chem.Phys.16, 21941(2014)

