Scientific Achievement
The relative concentrations of the critical species present in complex borohydride electrolytes were determined, quantifying solvation environment evolution and species formation as a function of which multivalent cation (Mg or Ca) is present.
Significance and Impact
Quantification of species populations serves as a rigorous validation of our previously hypothesized speciation pathways in borohydride electrolytes. An understanding of these pathways is essential for creating multivalent electrolytes required for practical, rechargeable calcium and magnesium metal batteries.
Research Details
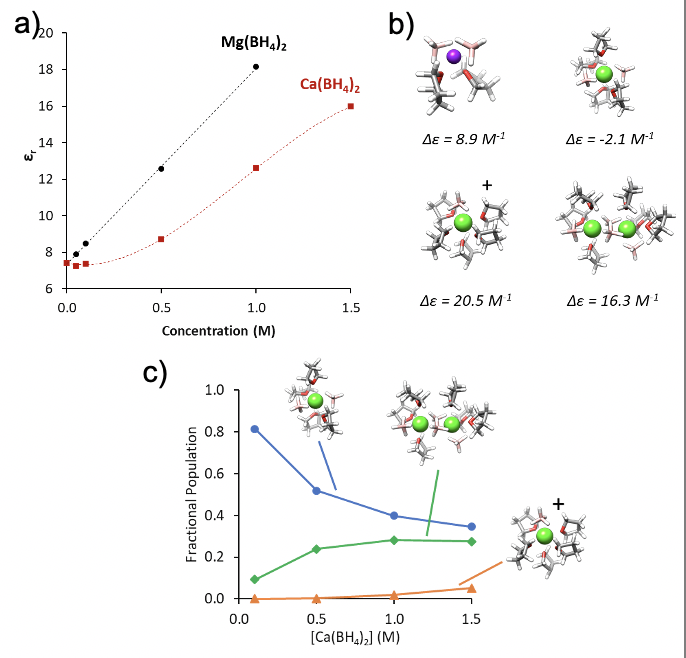
The dielectric constant of the Ca and Mg electrolyte are found to change differently with salt concentration using dielectric relaxation spectroscopy (DRS).
This class of electrolytes solvates the salt through formation of ionic clusters and molecular dynamics simulations show that dipoles of favored clusters are responsible for these different trends in dielectric constant.
We perform species quantification based on these dielectric constants and molecular structures, coupled with mobility measurements from nuclear magnetic resonance spectroscopy, revealing the differences in population trends for Ca and Mg and helping to explain the better performance of the Ca electrolyte relative to Mg.

