Scientific Achievement
We conduct SAXS study to investigate the solvation behavior of LiTFSI aqueous solutions in a wide range of concentration from 1 to 20 m. Also, the stability of these samples under different temperatures is studied by in situ SAXS measurements.
Significance and Impact
The paper shows that TFSI- solvated structure and TFSI- network can coexist at a particular concentration, and temperature changes will lead to one structure’s formation or disappearance. Also, the TFSI- network is the key to obtain a stable electrochemical window under relatively high temperatures.
Research Details
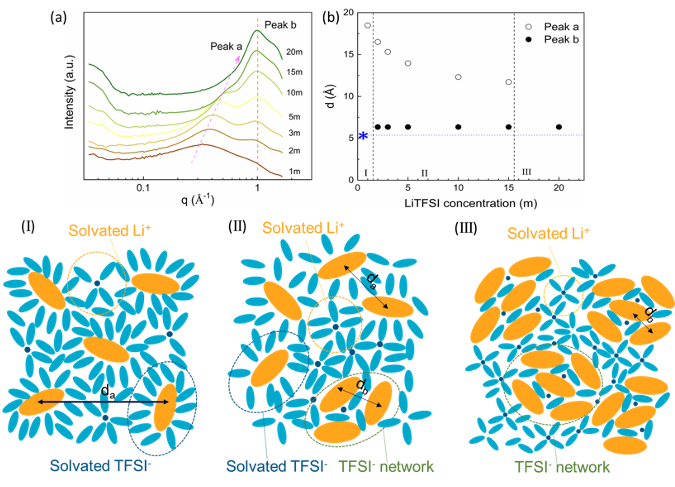
- TFSI- anions can form two different structures, TFSI- solvated structure and TFSI- network.
- At low concentrations, the TFSI- solvated structure is the main solvated structure. TFSI- solvated structure and TFSI- network coexist as the concentration increases to the medium concentration. At high concentrations, only the TFSI- network remains.
- Increasing temperature may promote the conversion between bulk water and interfacial water. The structural changes caused by the temperature are the reversible process.

