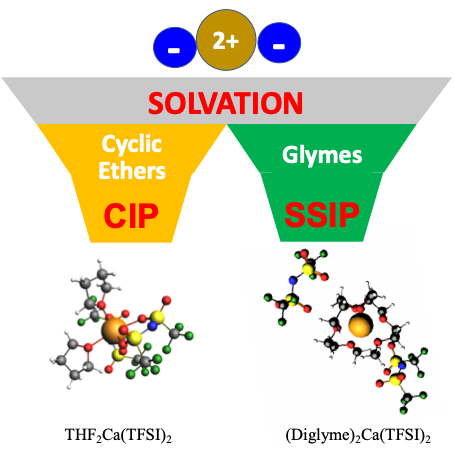
Scientific Achievement
Discovered and described how solvent structure and cation size influence cation−anion interactions, suggesting that they drive the formation of free ions in ethereal multivalent electrolytes.
Significance and Impact
These results provide a fundamental understanding of the physical chemistry in multivalent battery electrolytes and can be used as guidelines for the design of future secondary batteries.
Research Details
- Pulsed field gradient nuclear magnetic resonance and Raman spectroscopy experiments show that specific solvent−cation interactions are stronger for glymes than for cyclic ethers.
- Despite having a lower charge density, the larger-sized Ca2+ generally interacts more strongly with anions than Zn2+.
- Ionic conductivity, ΛM, and dielectric relaxation spectroscopy measurements reveal that second-shell ion correlations are prevalent in these solvents as are dipolar ion pairs, suggesting a general mechanism for de-correlating ion motion in relatively
well-dissociated multivalent electrolytes.

