Scientific Achievement
The interfacial structure of the water-in-salt electrolytes (WiSE) on a mica surface is investigated. Nanostructures of 1nm to 3 nm are observed. The size of the nanostructure varies depending on the concentration of the LiTFSI/H2O system.
Significance and Impact
Our study suggests the nanoheterogeneity of the WiSE at the solid/electrolyte interfacial region, and corresponds with previous computational and experimental studies on the bulk nanoherterogeneity of the electrolyte.
Research Details
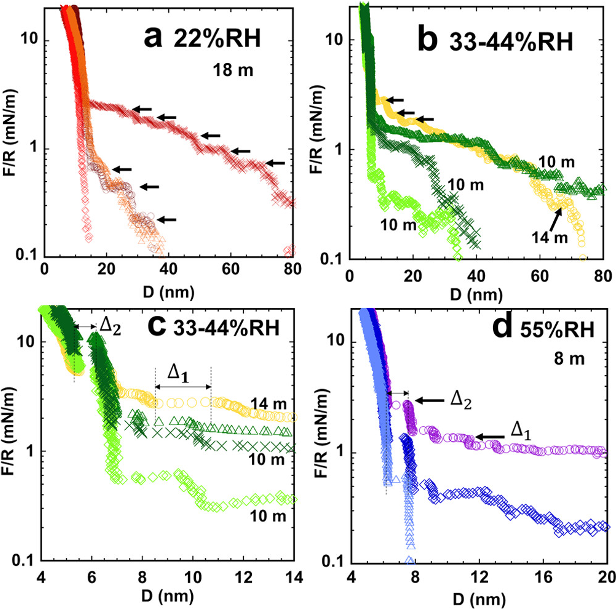
- WiSE/mica surface structure is studied with AFM and surface force apparatus (SFA).
- Smaller layered structures are observed in AFM (< 1nm), at a closer distance to the mica surface (D < 4 nm).
- Larger nanostructures are observed in SFA farther from the mica surface (D > 4 nm), ranging from 1 nm to 3 nm.
- The nanostructure size increases with higher concentration.
- In 18 m-21 m system, Li(H2O)x+, H2O, [Li(H2O)x]+[TFSI]-)y, and nanoclusters of 2nm-3nm are observed respectively from the mica surface to the bulk electrolyte.
- The interfacial structure exhibits a hysteresis for 8 m, possibly due to the breakdown of nanostructure with a lower salt-to-water ratio.

