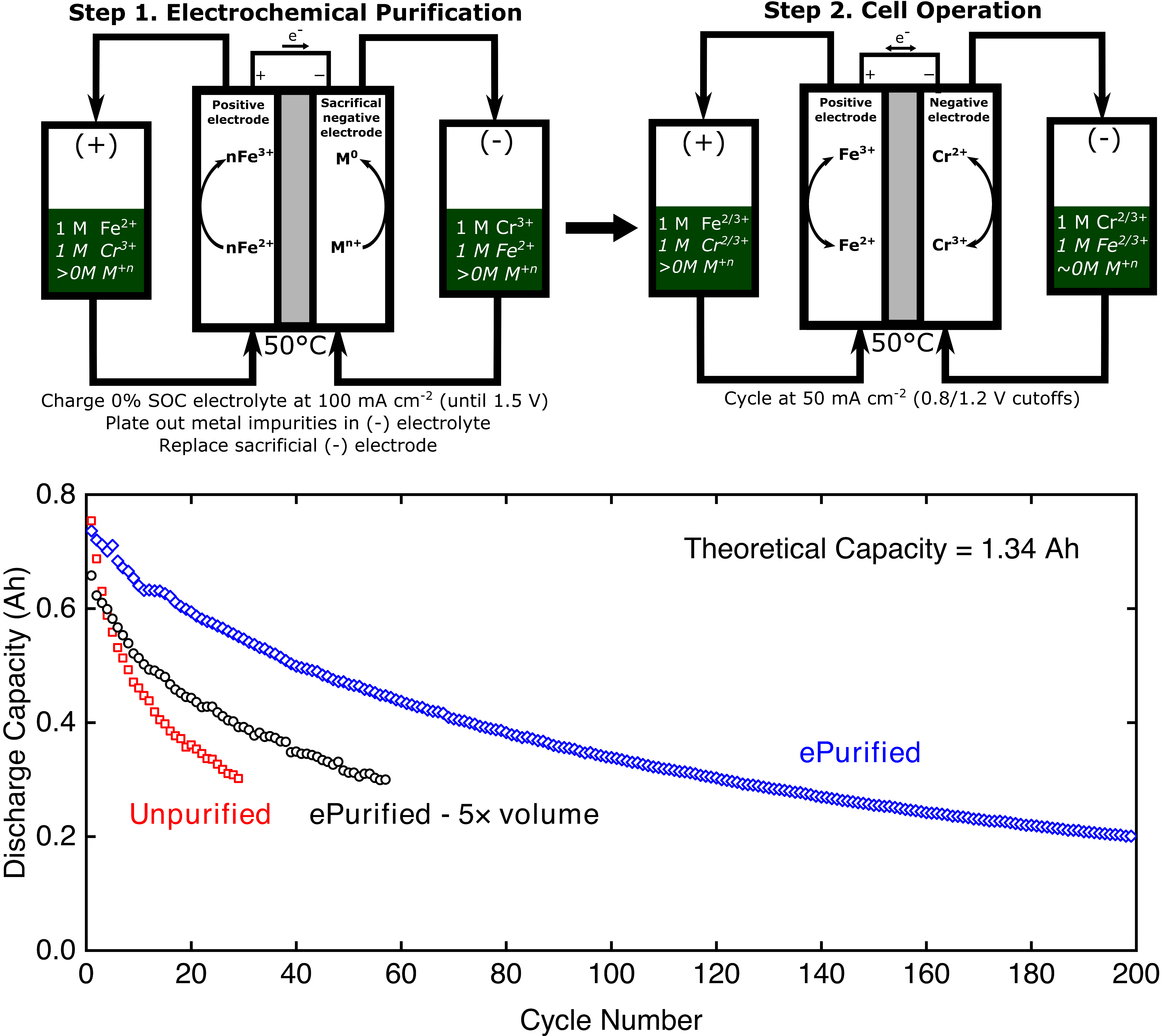
Scientific Achievement
An electrochemical purification method is used to mitigate the hydrogen evolution reaction (HER) in iron-chromium (Fe-Cr) redox flow batteries (RFBs), resulting in reduced capacity fade rates.
Significance and Impact
Fe-Cr RFBs hold promise due to their use of low-cost, abundant chemicals, but reductions in HER rates are needed to improve performance (efficiency, capacity fade). This work describes a purification method, electroplating of soluble impurities into a sacrificial electrode, that enables decay rates comparable to those obtained in cells with expensive catalysts or electrolyte additives.
Research Details
- The electrolyte-soluble impurities hypothesized to promote HER are removed via electrodeposition onto a sacrificial cathode in a temperature-controlled flow cell testbed (electrolytic process).
- The cell was dissembled after the purification step, the sacrificial electrodes were replaced, and the cell was re-assembled for cycling analysis.
- The protocol enables reduced capacity fade (ca. 5× slower) during galvanostatic cycling.
- A meta-analysis of cycling data in Fe-Cr RFB literature reveals a statistically significant association between coulombic efficiency and discharge capacity decay rate.

