Research Highlights
-
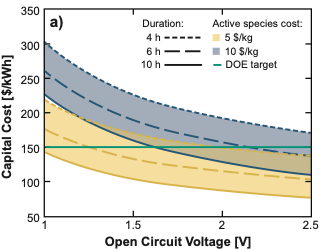
Untapped Potential: The Need and Opportunity for High-Voltage Aqueous Redox Flow Batteries
Prior studies of the techno-economic design space for aqueous redox flow batteries (AqRFBs) have almost exclusively focused on cell potentials ≤1.5 V, due, at least in part, to the belief that battery operation at higher cell potentials in not feasible due to electrolyte decomposition. However, through careful consideration of electrolyte composition, cell design, and operating practices, AqRFBs with OCVs >1.5 V can operate with minimal and/or manageable side reactions. Read More
-
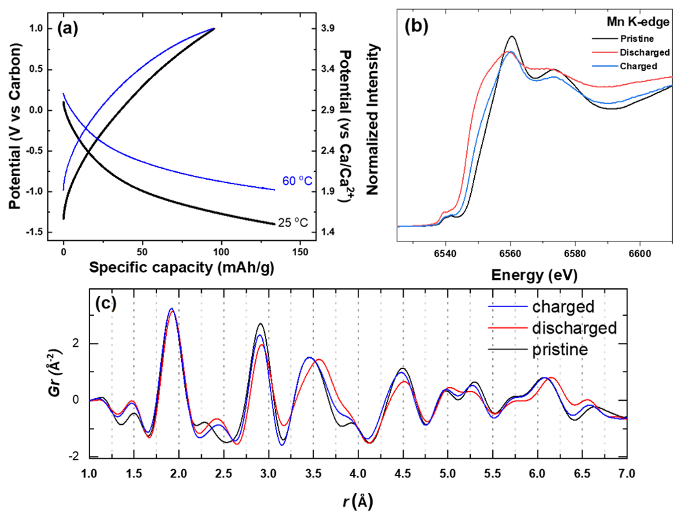
Intercalation of Ca into a Highly Defective Manganese Oxide at Room Temperature
Nanocrystals of layered MnOx containing a high concentration of atomic defects and lattice water are shown to have remarkable electrochemical activity towards Ca2+ , amounting to a capacity of ~130 mAh/g at room temperature. Multimodal characterization revealed the notable degree of intercalation by probing the structural, compositional and redox changes undertaken by the defective MnOx nanocrystals. Read More
-
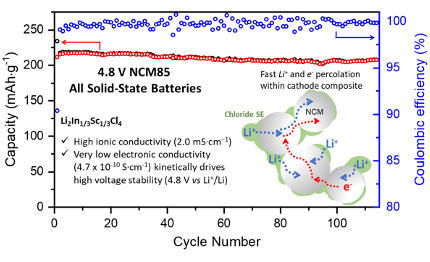
High areal capacity, long cycle life 4 V ceramic all-solid-state Li-ion batteries enabled by chloride solid electrolytes
Long-lasting, high-loading and high-voltage ASSBs with bare LiNi1-x-yCoxMnyO2/LiCoO2 and a new chloro-spinel solid electrolyte Li2In1/3Sc1/3Cl4 are demonstrated. The ultra-low electronic conductivity of Li2In1/3Sc1/3Cl4 drives a very wide “kinetic” electrochemical stability window (up to 4.8 V vs Li+/Li) that lies well above its thermodynamic stability limit (4.3 V vs Li+/Li). Read More
-
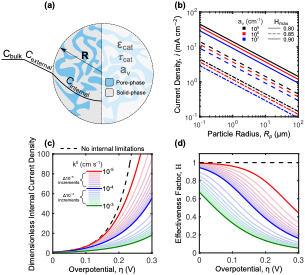
Methods–A Potential-Dependent Thiele Modulus to Quantify the Effectiveness of Porous Electrocatalysts
A generalizable potential-dependent Thiele modulus accounting for the relationship between electrochemical reaction kinetics and diffusion is presented for the design of porous catalyst materials for use in electrochemical reactors such as redox flow batteries. Read More
-
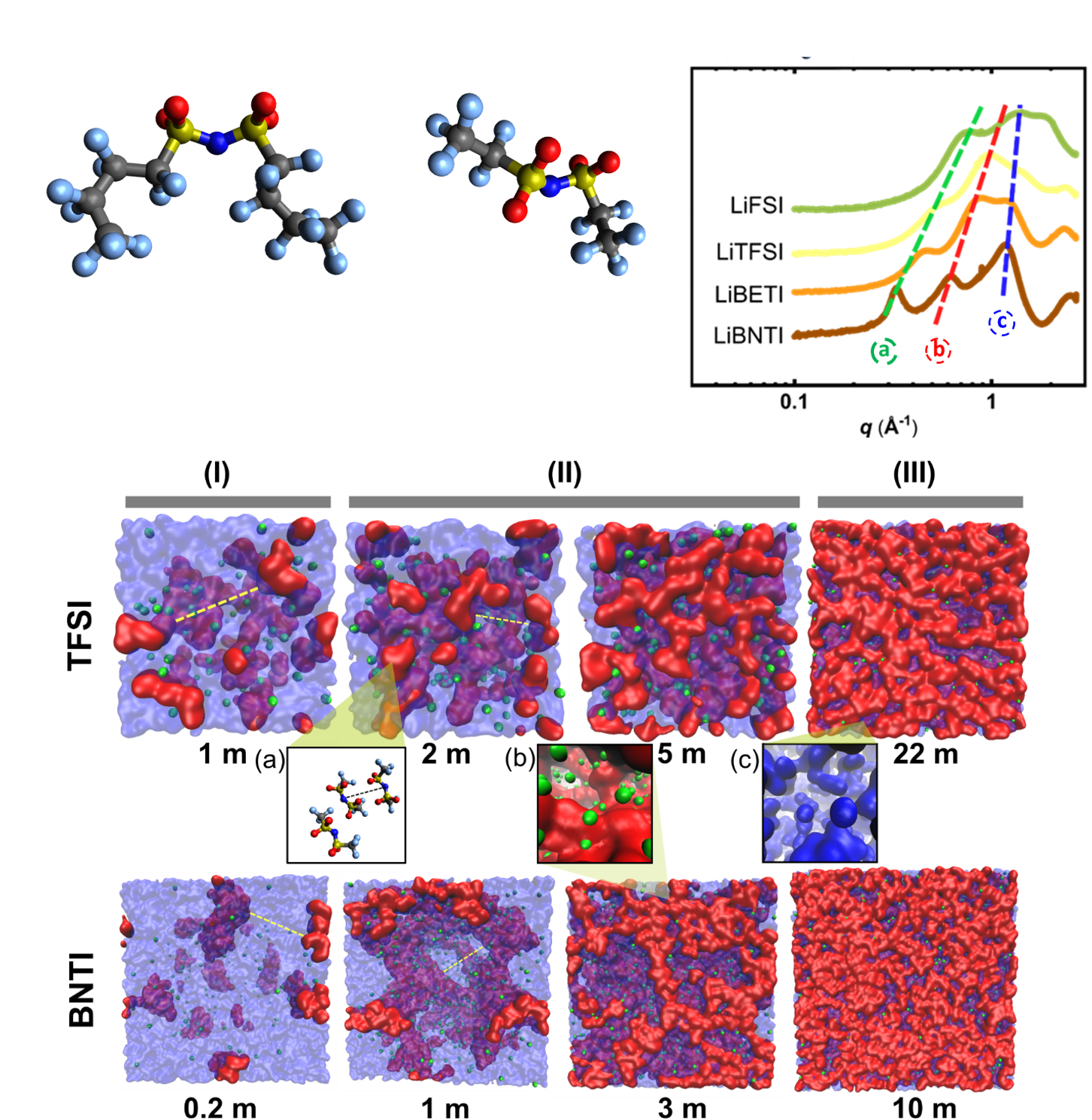
Insight into the nanostructure of “water in salt” solutions: a SAXS/WAXS study on imide-based lithium salts aqueous solutions
We studied the structures of a series of imide-based lithium aqueous solutions: bis(fluoro sulfonyl)imide (FSI), bis(trifluoromethane sulfonyl)imide (TFSI), bis(pentafluoroethane sulfonyl) imide (BETI), and bis(nonafluorobutane sulfonyl)imide (BNTI) at various concentrations through small angle X-ray scattering/wide angle X-ray scattering (SAXS/WAXS) and molecular dynamics (MD) simulations Read More
-
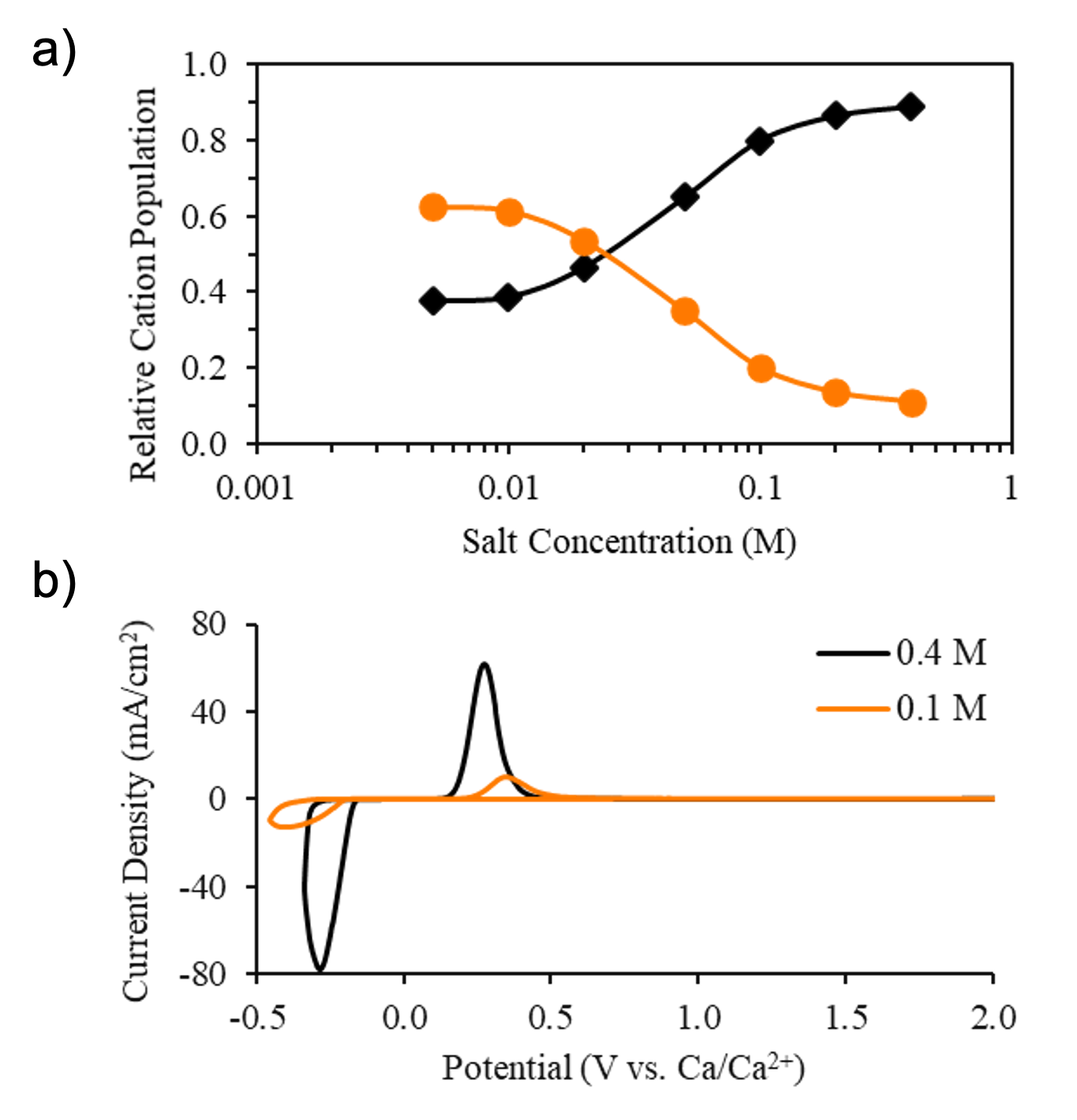
Concentration-Dependent Ion Correlations Impact the Electrochemical Behavior of Calcium Battery Electrolytes
The speciation trends of weakly-coordinating multivalent salts in ethereal solvents were established, providing a generalized understanding of how multivalent ion correlations impact electrolyte transport and stability. Read More
-
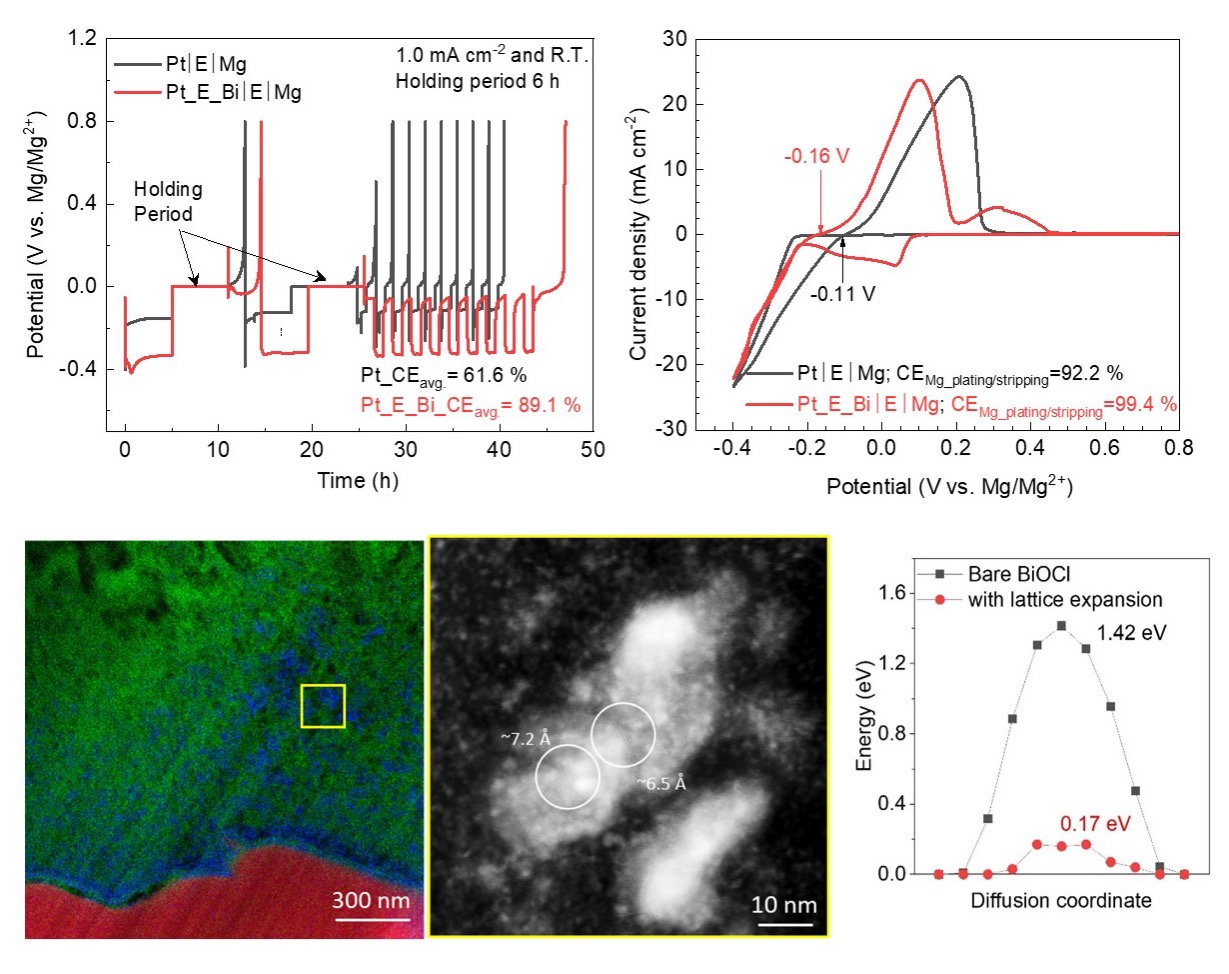
Enabling magnesium metal anodes via an electrochemically activated artificial interphase layer
The dynamics and instability of the Mg/electrolyte interface over extended time were revealed and an artificial interfacial layer with fast ion transfer kinetics was developed to suppress side reactions and thus improve electrochemical stability. Read More
-
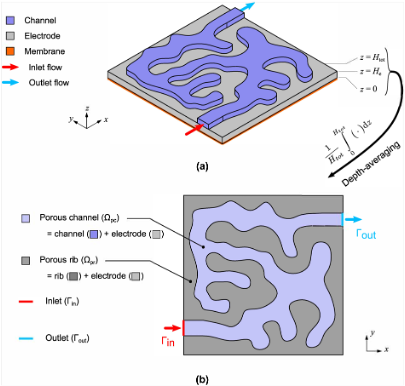
A generalized reduced fluid dynamic model for flow fields and electrodes in redox flow batteries
Depth-averaged fluid dynamics dramatically reduce the computation time involved in screening flow fields and porous electrodes (3D 2D), while retaining accuracy through a correction to the governing equations. Read More
-
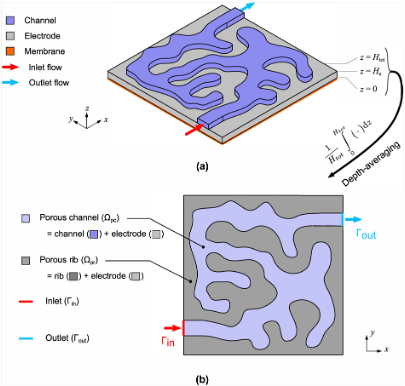
A generalized reduced fluid dynamic model for flow fields and electrodes in redox flow batteries
Depth-averaged fluid dynamics dramatically reduce the computation time involved in screening flow fields and porous electrodes (3D 2D), while retaining accuracy through a correction to the governing equations. Read More
-

Batteries: materials principles and characterization methods
This research and reference text provides an introduction to battery fundamentals, exploring some of the state-of-the-art characterization methods currently employed by the energy storage community. Read More
Latest Updates
-
You’re Invited - JCESR and Beyond: Translating the Basic Science of Batteries
Please join us at Argonne National Laboratory on Tuesday, April 4, 2023 for JCESR and Beyond: Translating the Basic Science of Batteries. Registration is now open. This in-person event will celebrate 10 years of research from the Joint Center… Read More
-
A Message from JCESR: In Memory of George Crabtree
It is with heavy hearts that we say goodbye to George Crabtree, a Senior Scientist and Distinguished Fellow at Argonne National Laboratory, and Director of the Joint Center for Energy Storage Research (JCESR), who passed away unexpectedly on January 23. Dr. Read More
-
Cyanopyridines As Extremely Low-Reduction-Potential Anolytes for Nonaqueous Redox Flow Batteries
Discovery of a cyanophenylpyridine derivative with a very low reduction potential and good stability during cycling. Read More
-
Characterizing Redoxmer – Electrode Kinetics Using a SECM-Based Spot Analysis Method
Identified asymmetries in electron transfer (ET) kinetics between the reduction and oxidation of ferrocene-based redoxmers by measuring the ET rate constants (kf/kb) as a function of electrode potential. Read More
-
Benzotriazoles as Low Potential Anolytes for Non-Aqueous Redox Flow Batteries
We developed an easy-to-synthesize benzotriazole-based anolyte with a high energy redox potential (-2.3 V vs Fc/Fc+) and high solubility that demonstrates stable electrochemical cycling performance. Read More

