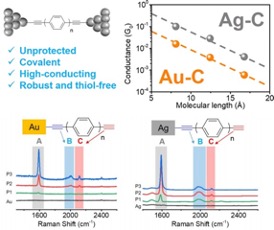
Scientific Achievement
Unprotected acetylene groups were found to form a simple new electrode-anchor pair (silver-acetylene) for forming robust, low resistance contacts for mediating or controlling molecule-electrode interactions.
Significance and Impact
This work highlights the importance of electrode-molecule interactions for mediating charge transport near the electrode surface. This work will facilitate an improved understanding of charge transport mechanisms and electron transfer rates for redoxmers in redox-active flow batteries.
Research Details
- We report the spontaneous formation of robust and covalent metal-molecule linkages using acetylene-terminated oligophenyls with Ag electrodes, resulting in high-conducting molecular linkages with significantly lower contact resistance (6 kΩ) than those with acetylene-Au (36 kΩ), S-Au (40 kΩ), and amine-Au contacts (189 kΩ).
- Different metal binding motifs result in lower Ag-C contact resistance compared to Au-C contacts

