Scientific Achievement
Nanocrystals of a few-layer manganese oxide are shown to have considerable electrochemical activity toward Mg2+ intercalation even at room temperature, where they delivered ~190 mAh/g in full Mg metal batteries.
Significance and Impact
These observations constitute a foundational step toward Mg batteries with high energy density, which require oxide cathodes. So far, all oxides explored have shown fundamental barriers toward extensive Mg2+ intercalation at room temperature, and, thus, very low capacity. This work bridges this gap, paving the way to new avenues of materials design.
Research Details
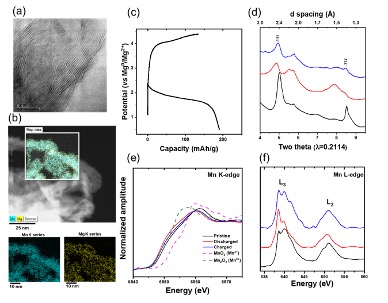
- Few-layer nanoscrolls of a manganese oxide showed high electrochemical activity in non-aqueous electrolytes developed by JCESR for full cells with Mg metal.
- High capacity was reached at a high potential of at ~1.9 V (vs Mg/Mg2+)
- A suite of analyses with techniques showing different chemical and structural sensitivity supported the existence and reversibility of Mg2+ intercalation into the oxide.
- Compared with other reports that showed no intercalation, our few-layer manganese oxide contained interlayer defects, which points at novel avenues of design of materials complexity tailored for Mg2+ intercalation.

