Scientific Achievement
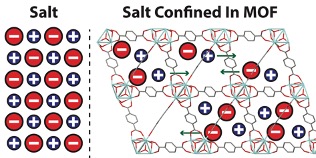
The nanoscale confinement of [NEt4][TFSI] in three isostructural MOFs enhanced the conductivity of composites relative to neat [NEt4][TFSI] by up to a factor of 50. The conductivity increases with the increase in pore size and maximum conductivity was achieved with a salt loading slightly less than that required for complete filling of the MOF pores.
Significance and Impact
Spatially confining [NEt4][TFSI] in isoreticular, microporous, Zr-based stable and insulating MOFs leads to pore-size and loading-dependent enhancement of ionic conductivity. The melt loading of salt under solvent-free conditions provides a means to study the solid-state ionic conductivity of the salt-MOF composites in the absence of competing solvent-mediated pathways. [NEt4][TFSI] serves as a model for other salts such as LiTFSI, Mg(TFSI)2, etc. that are of interest for solid electrolytes in metal-ion batteries.
Research Details
- The salt loading was monitored via PXRD and DSC.
- AC electrochemical impedance spectroscopy was used to determine the ionic conductivity of several MOF-[NEt4][TFSI] composites, with varying composition, as a function of temperature. The conductivity for all composites increased as a function of temperature.

