Scientific Achievement
Three quinone-based organic electrodes were tested in nonaqueous Zn-batteries using different salts and solvents. Mechanistic studies on the electrode kinetics were then performed.
Significance and Impact
A major hurdle to multivalent ion battery technology is the cathode systems available for facile intercalation kinetics and stability. Quinone-based electrodes have been reported to show favorable intercalation kinetics in multivalent ion batteries. Understanding their activity in Zn-ion systems will be important in future efforts toward tunability of organic electrode systems and conditions for Zn-ion batteries.
Research Details
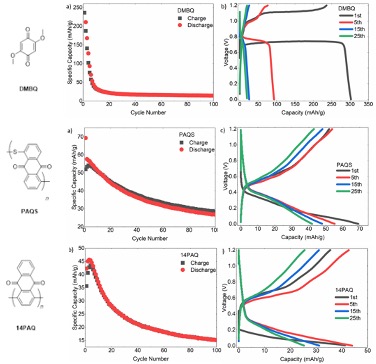
- 2,5-Dimethoxybenzoquinone (DMBQ), polyanthraquinone sulfide (PAQS), and 1-4-polyanthraquinone (14PAQ) were used as electrode materials
- Electrolyte and electrode choices were refined using electrochemical tests and post-mortem analyses such as XRD and EDS
- Zn(TFSI)2/acetonitrile exhibited the best performance
- DMBQ displayed typical faradaic behavior, while the polymeric derivatives both showed slow charge transfer
- Slow capacity fade shows dissolution of cathode material was mitigated in polymeric systems

