Scientific Achievement
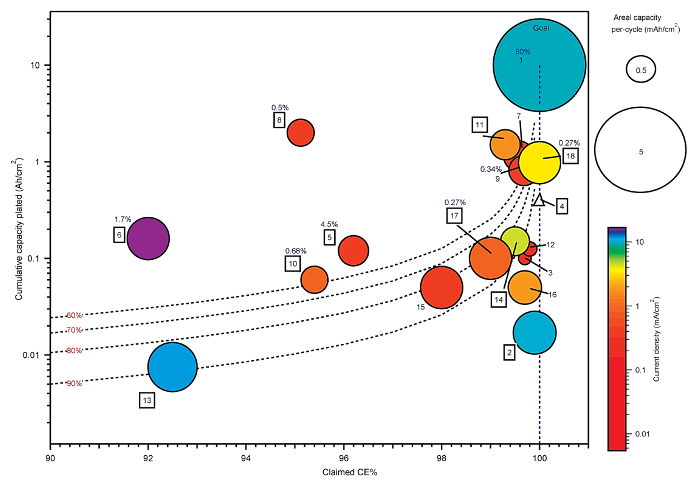
The first comprehensive comparison of the ~20 published studies evaluating Zn metal reversibility for rechargeable batteries shows significant variability in published test methods and parameters, and poor alignment toward commercial targets for zinc metal batteries.
Significance and Impact
Pending adoption by the field, the conclusions and recommended methods from this Perspective have the potential to unify development efforts for zinc metal batteries, streamlining commercialization of this promising system and eventually enabling access to a potentially safer, more sustainable battery technology than Li-based systems with improved energy per volume.
Research Details
- More than half of the studies published on electrochemical reversibility of Zn claim a coulombic efficiency higher than 99%, yet rechargeable zinc metal batteries are still far from commercialization.
- The use of cyclic voltammetry as a probe for metal plating/stripping reversibility is strongly discouraged in favor of galvanostatic methods, which more closely emulate conditions in real cells.

