Scientific Achievement
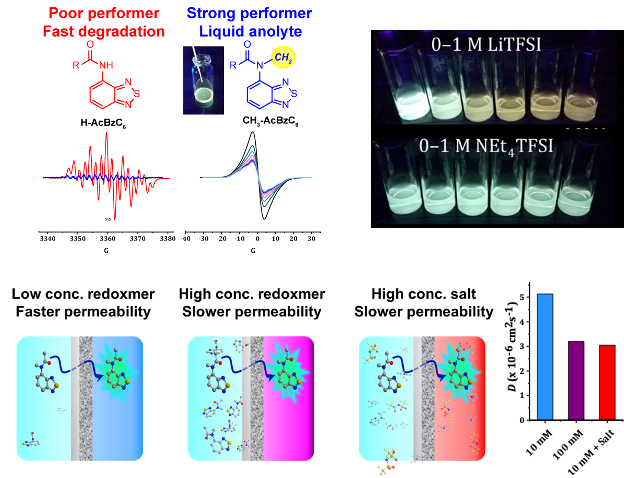
Organic redox-active molecules (redoxmers) are popular for flow battery fluids. Their structural variability comes with other properties that may be tailored for function. We discovered that fluorescence emission can be engineered into redoxmers, which allows real-time tracing of species crossover in a liquid flow cell, a key state of health (SOH) metric. The extracted permeability values show dependence on composition of electrolytes such as salt or redoxmer concentrations.
Significance and Impact
Responsiveness is a critical feature for redoxmers that can enable rapid sensing of SOH and materials restoration for long-term performance in a demanding battery environment. “Self-reporting” fluorescent redoxmers perform their battery function and have another property that does not interfere with battery operation. The findings serve as a successful example of multi-property redoxmer design as well as implementing responsiveness in flow cells, which may prompt broader interest across JCESR and flow battery field.
Research Detail
- High-yielding syntheses of fluorescent 2,1,3-benzothiadiazole redoxmer molecules H-AcBzC6 and CH3-AcBzC6
- Electrochemical stability depends on chemical structure
- Strong ion-redoxmer interactions in lithium-based electrolytes
- Crossover was compared for 10 mM redoxmer, 100 mM redoxmer and 10 mM redoxmer + 1 M NEt4TFSI salt; the latter two decrease permeability of the redoxmer through the flow cell design

