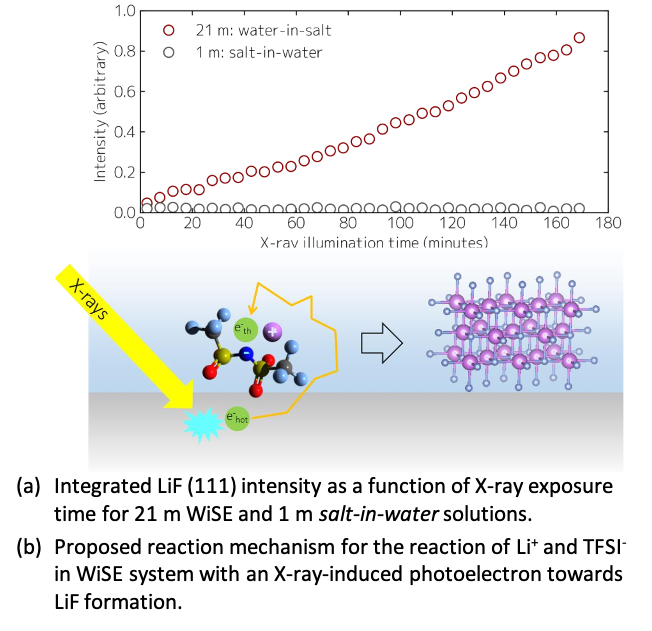
Scientific Achievement
LiTFSI/H2O electrolyte interfacial decomposition pathways in the “water-in-salt” (WiSE) and “salt in-water” regimes are investigated using synchrotron X-rays. The resultant photoelectron-induced reduction towards LiF formation was revealed to be occur among closely contact ion-pairs adjacent to the interface. This supports present models behind the WiSE concept.
Significance and Impact
This work shows that interfacial speciation determines interfacial chemistry on solid surfaces in WiSE, and therefore might dictate the eventual electrolyte stability window. The observed radiation-induced phenomena could also occur in other highly concentrated electrolytes that contain fluorinated species such as conventional non-aqueous electrolytes, polymer electrolytes, ionic liquid, and gel electrolytes.
Research Details
- WiSE: 21 m LiTFSI in water (and its later progenies).
- X-ray chemistry-X-ray probe: investigate in situ the structural properties of in situ formed surface films.
- X-ray photoelectron spectroscopy (XPS), scanning electron microscopy energy-dispersive X-ray spectroscopy (SEM-EDX), and optical microscopy: investigate the surface film’s morphology and chemistry.

