Scientific Achievement
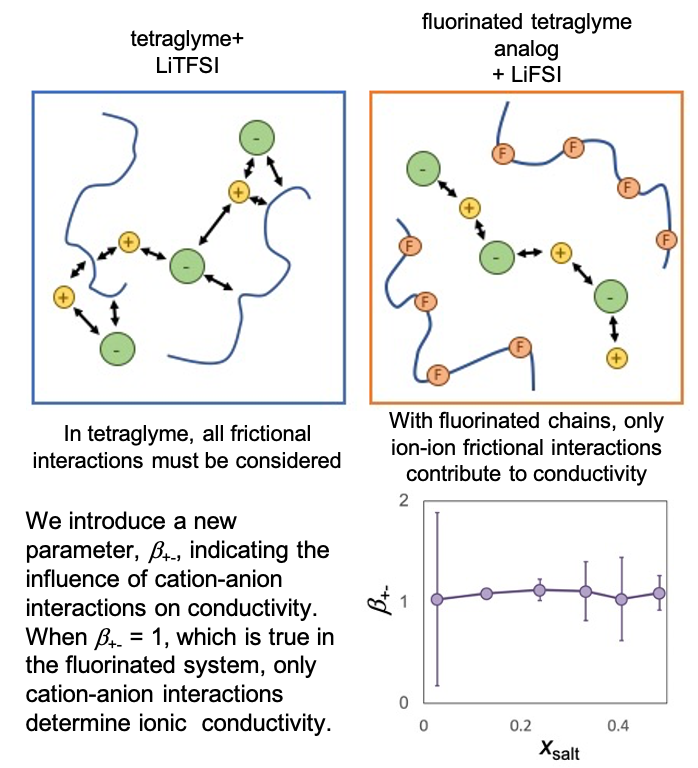
This work introduces a new way to analyze the importance of ion-solvent and cation-anion frictional interactions, and illustrates a new regime in which cation-anion interactions are the sole determinant of ionic conductivity.
Significance and Impact
It is generally assumed that ionic conductivity is governed by the self-diffusion of the ions, which in turn is influenced by frictional interactions between the ions and the solvent, as proposed by Nernst and Einstein. We show the existence of an entirely different regime wherein friction between the cation and anion dominates.
Research Details
- Full electrochemical characterization was performed on two systems which differ primarily in that one is fluorinated.
- In the fluorinated system, anion-solvent interactions determine transference number, while cation-anion interactions determine conductivity. In the hydrogenated system, all interactions must be included.
In both systems, there is no direct relationship between conductivity and ion self-diffusion coefficients

